Scroll to:
Unravelling the neurochemical maze: neurotransmitters, neuropeptides and novel drug modes of action based on epilepsy pathophysiology
https://doi.org/10.17749/2077-8333/epi.par.con.2023.152
Abstract
The brain is extremely complicated three dimensional structures made up of interconnected neurons and neuroglia cells. It entails all type of functions of our body whether we are healthy or in disease conditions. Brain is accountable for our connectivity with the surroundings; all this is performed by an organized and systemic electrical activity of neurons by which they communicate messages to and from the brain. The abnormal electrical activity leading to the intense outburst of impulses, results in the development of epilepsy. Epilepsy is typified by recurrent, unprovoked seizures as a result excessive, hypersynchronous discharge of neurons occurs in the brain. Nearly 1% of the population throughout the worldwide is suffering from epilepsy and almost 75% begins at childhood. The patients almost one third are resistant to current available antiepileptic drugs. We don’t have the deep knowledge of the pathophysiology of the disease which can prove useful in further research for drugs with new mechanisms of action for diseases. This paper covers the role various neurotransmitters and neuropeptides in the pathophysiology of epilepsy. Our objective is to introduce the scientists with that aspect of the disease which may prove useful for further development of new drugs of epilepsy to overcome the resistance shown by the patientsorithm.
Keywords
For citations:
Dhall M., Kadian R., Sharma P., Hooda A., Kumar P., Mudgal P., Singh K., Arya A., Rani N. Unravelling the neurochemical maze: neurotransmitters, neuropeptides and novel drug modes of action based on epilepsy pathophysiology. Epilepsy and paroxysmal conditions. 2023;15(3):282–293. https://doi.org/10.17749/2077-8333/epi.par.con.2023.152
INTRODUCTION / ВВЕДЕНИЕ
Epilepsy is typified by the tendency to have repeated seizures, which in Latin means ‘to strike’ as a result of sudden synchronous discharges in cerebral cortical neurons leading to troubled movements, sensation and consciousness. In epilepsy, the main focuses, there is an imbalance between the excitatory neurotransmitter such as dopamine, noradrenalin and glutamate when compare with inhibitory such as of serotonin and gamma-aminobutyric acid (GABA) via GABAA receptors, modified sodium, chloride, calcium and potassium currents.
Epilepsy is usually identified through a combination of electroencephalographic (EEG) testing and a complete examination of the state of a patient by a skilled expert of epilepsy disorder. Adults are identified with disorder merely when they have revealed recurring incidents of seizures, those who have encounter a seizure episode just one time are not thought to be epileptic [1]. Following diagnosis, patients are categorized based on the type of seizures they are exhibiting and after this treatment is given. Seizures might stay restricted to their region of starting point (“focal” or “partial” seizures) or extend to the complete hemispheres of cerebrum (“generalized” seizures).
Symptoms of the seizures firmly depend on the portions of brain which are influenced by hyperactivity. Seizures have been usually described as anunevenness between dopaminergic and serotonergic neurons and between glutamatergic (excitatory) and GABAergic (inhibitory) transmission. The neuropeptides perform an important function in modifying the brain excitability by modulating the role neurotransmission such as dopamine and serotonin. Many neuropeptides have been described to perform a function as an anticonvulsant. Irregularity in the concentration of certain neuropeptides is also reported in patients suffering from epilepsy. The classification of types of epileptic seizures are represented in the Figure 1 [3].

Figure 1. Classification of epileptic seizures types (adapted from [3])
Рисунок 1. Классификация типов эпилептических приступов (адаптировано из [3])
The living life of most adults with epilepsy still is badly influenced due to lack of awareness, support, not timely diagnosis, improper and irregular treatment, illiteracy, regulation, and improper research [2]. The deep study of the role of neurotransmitters, neuropeptides, and their receptors can serve as an important target for the development of new antileptic drugs. In this review article it has been updated the role of altered neurotransmitters and neuropeptides in epilepsy. Currently pharmacological treatment is effective only in two third patients and thus new drug targets are required [3][4].
PATHOPHYSIOLOGY / ПАТОФИЗИОЛОГИЯ
GABAA receptors, which regulate excitability, and GABA, an inhibitory neurotransmitter performs a significant part in the pathophysiology and progress of epilepsy [5][6]. Serotonin receptors by altering binding functions have been reported to ensure a robust association with anxiety and epilepsy. Currently, the Ca2+ channels which regulate the secretion at the synapse of neurotransmitters is anticipated to be concerned in the beginning of anxiety in the patients of epilepsy. The disturbance in the inhibitory control or imbalance of both is recognized to perform a chief regulatory role in disease progress [7].
The interval of excitation due to glutamate can be controlled by clearing the synaptic cleft which controls the extracellular levels, thus thwarting hyperexcitability. So the neurotransmitter transport can be seen as the important possible mechanism to decrease the concentration of the excitatory neurotransmitter in the synaptic cleft [8]. There are five types of glutamate receptors which have been identified some of them are reported to reside on neurons and the rest on to glial membranes [9]. Glutamate transporters help in the removal of excessive glutamate from the synaptic cleft resulting in synaptic inactivation [10].
By exploring the role of neurotransmitter transporters and their role in excitatory and inhibitory neurotransmission and the participation of the preeminent level of glutamate-glutamine shuttle operation for constant epileptic activity, may expose the novel perceptions that suggest that the therapeutic variations in the supply of neurotransmitters may cause reduction in seizure activities [11].
Ion channels interaction / Взаимодействие ионных каналов
Voltage gated ion channels proper functioning is very important for the synchronous generation and propagation of action potential. So they definitely perform a function in the origin and mitigation of epilepsy. Mainly the channel involved are voltage gated sodium channels as we see that many antiepileptic drugs (AEDs) also act by suppressing these channels [12]. Other ionic channels involve are potassium, chloride, and calcium [13]. Epileptogenic agents act through voltage-gated ion channels by modifying excitatory and inhibitory neurotransmission [14].
Glutamate is the chief excitatory amino acid neuro-transmitter present in the brain. The glutamate receptors can be present presynaptically, postsynaptically, and on specific types of glial cells. The ionotropic subcategories are namely alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA), N-methyl-D-aspartate (NMDA) and kainate receptors, are considered to perform a main function in epilepsy [15]. Agonist of these receptors induce epilepsy and antagonist suppress seizures. GABAB receptors which present postsynaptically in the neurons act as inhibitory receptors when treated with their agonists suppress the seizures in epilepsy [15].
Calcineurin / Кальциневрин
Calcineurin (CaN) is a calcium-calmodulin-dependent pervasive protein phosphatase. It curb NMDA receptor activity and synaptic plasticity [16]. Cerebral cortex, hippocampus, and cerebellum are the main area of the brain where this phosphatase can be found in abundance [17]. Several animal models confirm the relation between CaN and epileptic seizures. It has been studied that the CaN expression was found to be enhanced in epileptic patients [18][19]. Calcineurin inhibitors namely FK-506 and cyclosporin A found to show neuroprotective effect. In status epilepticus CaN activity has been found to be enhanced in both hippocampus and cortex, further FK-506 and cyclosporin A both shown to impede the progression of kindling [20]. The pathophysiologic mechanisms of seizures are represented in Figure 2 [7][19].
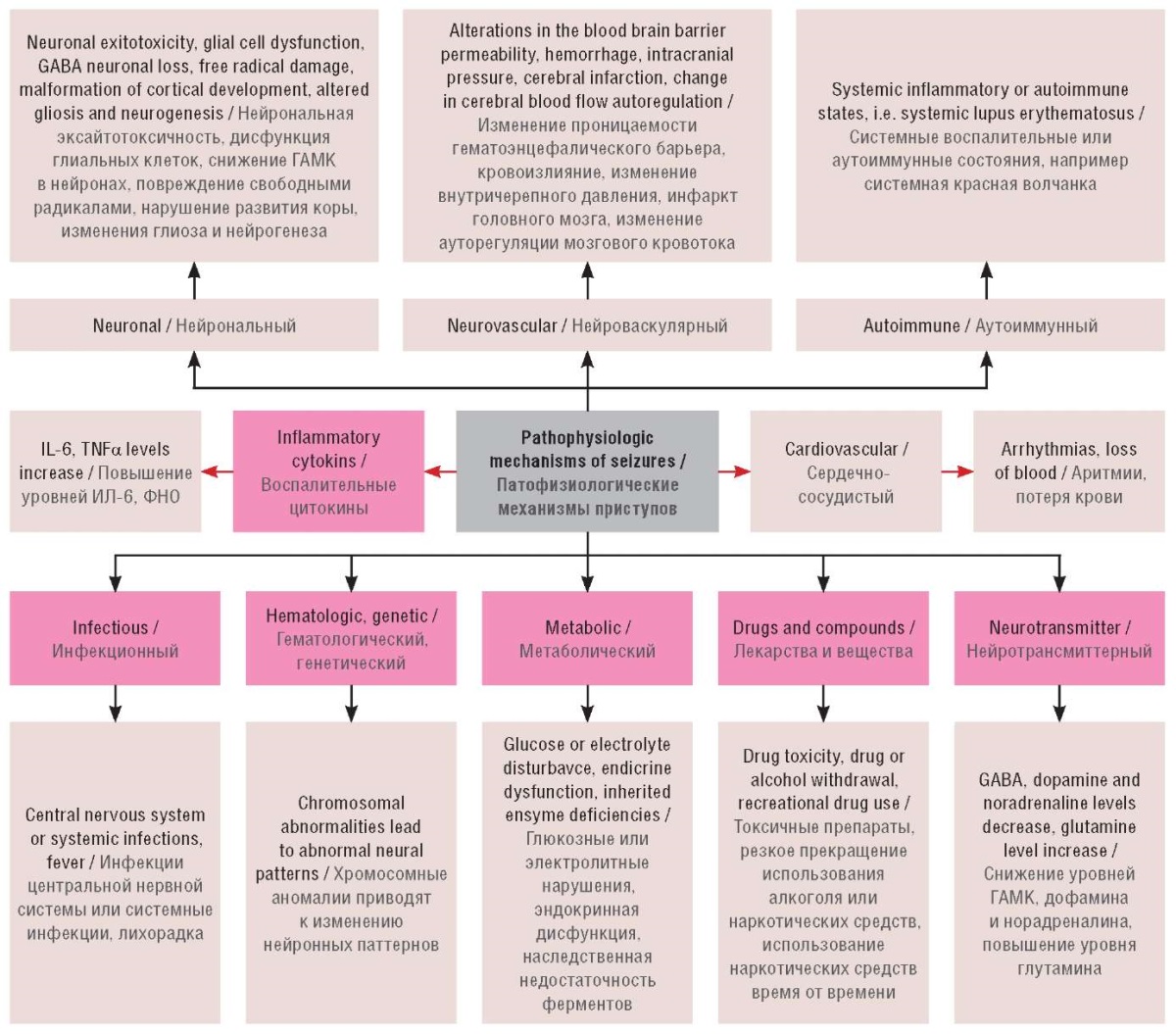
Figure 2. Pathophysiologic mechanisms of seizures (adapted from [7][19]).
GABA – gamma-aminobutyric acid;
IL – interleukin;
TNF – tumor necrosis factor
Рисунок 2. Патофизиологические механизмы приступов (адаптировано из [7][19]).
ГАМК – гамма-аминомасляная кислота;
ИЛ – интерлейкин;
ФНО – фактор некроза опухоли
Extra synaptic transmission in epilepsy / Внесинаптическая передача при эпилепсии
There are many studies which gives evidence for the involvement of synaptic transmission disturbances of excitatory and inhibitory neurotransmitters in epilepsy. Recently, it has been evident these receptors are also expressed extrasynaptically [20]. Main extrasynaptic neurotransmitter involved are glutamate and GABA [21]. Certain ionotropic glutamate receptor are present extracellularly. The main sites where they are found to be located are cell stomata and piramidal cells. The lower level of GABA is thought to always present in extrasynaptic spaces, the receptor recognized is GABA A subtype and are specifically responsible for tonic inhibition. But recently it has been hypothesized that extra cellular receptors have a distinct existence and have a specific role in various brain functions [22]. Thus, these receptors can be further explored for new treatment strategies.
Intracellular signaling pathways / Внутриклеточные сигнальные пути
Certain recent studies gives the evidences of an enhanced level of extracellular regulated kinase (ERK) at the time of spontaneous seizure in both human and animals [23]. These ERK kinases are copiously directed in the hippocampus, although it is the part of the brain involved mainly in memory and learning but most epileptogenic also [24]. These pathways are also activated during pathological conditions namely epilepsy and brain ischaemia. p38 and ERK are included in hippocampal seizure capable to initiate epilepsy in certain animal models [25].
NEUROTRANSMITTERS INVOLVED IN EPILEPSY / НЕЙРОТРАНСМИТТЕРЫ, УЧАСТВУЮЩИЕ В РАЗВИТИИ ЭПИЛЕПСИИ
Hippocampus is the main site for epileptogenic activity: GABA an inhibitory neurotransmitter and serotonin undergoes decreased activity and glutamate and catecholamines shows hyperactivity. The glutamate exerting an excitotoxic action at postsynaptic neuron [26].
Gamma-aminobutyric acid / Гамма-аминомасляная кислота
GABA is presynaptic inhibitory neurotransmitter having a vast distribution throughout the whole nervous system act mainly via GABAA receptors [27]. At the developmental stage of brain GABAA receptors initially function as excitatory and depolarizing present postsynaptically, after words becomes inhibitory hyperpolarizing and location is presynaptic [27]. Several of GABA agonist drugs are anticonvulsant, whereas GABA antagonists are mainly proconvulsant. Reduction of GABA synthesis is often epileptogenic. Drugs that enhance GABA-mediated inhibition and that enhances synaptic GABA are anticonvulsant [22][28][29].
Glutamate / Глутамат
Glutamate being the most ardent excitatory neuro-transmitter, its role in epilepsy has been attempted to be studied. Seizures results in elevations in extracellular glutamate, alterations in glial and neuronal manifestation of glutamate receptors and uptake transporters accounting to excitotoxic impairment. It has been studied that metabotropic glutamte receptors perform a significant function in modulating the actions depicted by ionotropic receptors in modifying the action of the epileptic focus. After pilocarpine-induced seizures, it has been found that there is down-regulation of presynaptic mGluR2/3 expression, which may promote enhanced excitability [30][31].
Noradrenaline / Норадреналин
The noradrenalin system is concerned in the pathophysiology of several brain disorders. In the brain this neurotransmitter is involved in the process of consciousness, sleep, learning and its reminiscence. The decrease in the concentration of this neurotransmitter seems to enhance epileptic activity and enhanced concentration seems to inhibit it. Mainly antiepileptic activity is considered to be through the involvement of its alpha 2 receptors. Beyond this seizure risk is associated with certain brain disorders which are related to monoaminergic disfunctions [32].
Dopamine / Дофамин
The limbic system related seizures are mainly influenced by Dopamine modulations. Functional control of dopamine is reported to be disturbed or might also be due to improper expression of certain dopaminergic receptors. In contrast, certain antiparkinsonian drugs stimulate D2 receptors. The decrease in calcium level affects the dopamine synthesis leading to epileptic convulsions without any disturbances of a dopamine receptor [33].
Serotonin / Серотонин
It is an old observation that depression and epilepsy runs side by side. The depression is common in temporal lobe epileptic patients. Psychiatric patients are also at higher risk of epilepsy.Serotonin along with other neurotransmitters maintains a balance between an inhibitory and excitatory system in cortex [34]. The evidences provided by the animal model regarding the involvement of serotonin and its receptors might act as a potential novel target for a new category of drugs for epilepsy with new mechanism [35].
Acetylcholine / Ацетилхолин
Destruction of the nicotinic receptors of the acetylcholine neurons present in the pedunculopontine tegmental nucleus are reported to involve in epilepsy. Certain acetylcholinesterase inhibitor drugs eg. soman lead to status epilepticus and seizures. These drugs enhances brain acetylcholine levels [36]. By report of literature, we can assume the association of the cholinergic system in the production of seizures and modification of key proteins associated in the cholinergic system leading to episodes of seizures. Further research can be done on the various cholinergic receptor systems regarding its involvement in epilepsy and new targets for development of newer drugs [37].
Other neuroactive substances / Другие нейроактивные вещества
The stimulation of inhibitory presynaptic adenosine A1 receptors has been reported to show a protective effect for neurons. While the barrier of excitatory postsynaptic adenosine A2 receptors depicts a remedial response in the above disorders [23].
NEUROPEPTIDES / НЕЙРОПЕПТИДЫ
The neuropeptides are basically signaling molecules just like neurotransmitters but with long half-life as compared to neurotransmitters (act only for milliseconds), thus have a long time effect on neuronal activity and modulate the seizure threshold. At present only adrenocorticotropic hormone (ACTH) and thyrotropin-releasing hormone (TRH) is being popular in clinical practice for epilepsy therapy. Certain neuropeptides have seizure suppressing and the other have proconvulsive properties [38][39].
Adrenocorticotropic and corticotrophin-releasing hormones / Адренокортикотропный гормон и кортикотропин-рилизинг-гормон
ACTH commonly known as corticotropin acts by binding the melanocortin receptors, nowadays most customarily used drug for treatment of infantile spasms. The etiology is based on the hypothesis of stress mediated release of corticotrophin-releasing hormone (CRH) neuropeptide in limbic regions of the brain. Origin place of CRH-induced seizures is amygdala spreading up to the hippocampus, here the expression of CRH receptors causes hyperexcitability by repression of after hyperpolarisation and potentiation of glutamatergic neurotransmission. ACTH mitigates the infantile spam by minimizing the production and secretion of CRH. ACTH, although is recommended therapy but with a lot of side effects so further research is required [40–42].
Dynorphin / Динорфин
Dynorphins are endorphins that act through opioid receptors most actions are κ-receptor mediated. These peptides act on presynaptic receptors and inhibit the release of excitatory amino acids to reduce excitability [43].
Galanin / Галанин
Several studies have reported the role of galanine neuropeptide in the epilepsy. In status epilepticus, it has been reported that there is severe depletion of galanin mainly from the hippocampus region of brain. Galanine causes presynaptic inhibition of glutamatergic neurotransmission. Galanin is also interacts with serotonin. Serotonin in the hippocampus shows anticonvulsant effect. Thus, Galanin can be considered as a future target for research of new anticonvulsant therapy [44]. The neurotransmitters alterations in epilepsy are mentioned in Table 1.
Table 1. Neurotransmitter alterations in epilepsy
Таблица 1. Нейротрансмиттерные изменения при эпилепсии
|
Neurotransmitter / Нейротрансмиттер |
Receptors involved / |
Effect in epileptogenesis / Влияние на эпилептогенез |
Reference / Источник |
|
GABA / ГАМК |
GABAA / ГАМКA |
Shows presynaptic inhibitory actions / |
[45] |
|
Glutamate / |
NMDA |
Imbalance in the inhibitory and excitatory neurotransmitters in the hippocampus as a result of brain injuries or status epilepticus / |
[46] |
|
Noradrenaline / |
Alpha 2 / Альфа 2 |
At low concentration exerts proconvulsant effects, and anticonvulsant effects at high concentrations in the hippocampus / |
[47] |
|
Dopamine / |
D1, D2 |
Frontal and parietal cortices is rich in D2-like receptors (to a lesser extent CA3 area of the hippocampus) in status epilepticus and genetic epilepcy / |
[48] |
|
Serotonin / |
5-HT |
Modulating effect upon epileptogenesis / |
[49] |
|
Acetylcholine / |
nACh alpha 7 / |
Enhances inhibitory GABAergic neurotransmission / |
[50] |
Note. GABA – gamma-aminobutyric acid;
NMDA – N-methyl-D-aspartate;
HT – hydroxytryptamine;
nACh – nicotinic acetylcholine receptor.
Примечание. ГАМК – гамма-аминомасляная кислота;
NMDA (англ. N-methyl-D-aspartate) – N-метил-D-аспартат;
HT (англ. hydroxytryptamine) – гидрокситриптамин;
nACh (англ. nicotinic acetylcholine receptor) –
никотиновый ацетилхолиновый рецептор.
Neuropeptide Y / Нейропептид Y
It is most abundant in GABAergic interneurons of central nervous system and hippocampus, act through Y1-5 receptors, although the predominant action is on Y1, 2 and 5 [51]. It has been reported by animal studies and also in epileptic patients that Neuropeptide Y concentration is increased during seizure. It is also studied that activation of Y2 and Y5 receptors and blockage of Y1 receptors helps to slow down epileptic activity.Thus, Y2 and Y5 agonists and Y1 antagonist can be explored for epilepsy treatment [51].
Somatostatin / Соматостатин
Somatostatin peptide is generally expressed in GABA interneurons in the hippocampus and are of two types 14 and 28 depending upon the number of amino acid present. Somatostatin act through five distinct receptors named SST 1–5, where receptor 2-4 are considered to have anticonvulsant action. These receptors are located on granular cells and control the excitatory input that comes to hippocampus. It has been reported in various models of animals of temporal lobe epilepsy that the density of somatostatin interneurons decreases after seizure [52][53].
Substance P / Вещество Р
Substance P, basically an element of the tachykinin family was investigated for pain and nociception. It acts at the neurokinin1 receptor, by reducing inward rectifying potassium currents. Substance P has been reported to show pro-epileptic effects. During intense status epilepticus, peptides changes their expression within the hippocampus [54–57].
Thyrotropin-releasing hormone / Тиреотропин-рилизинг-гормон
TRH, a tripeptide released from hypothalamus is also depicted in further brain portions namely cerebral cortex, amygdala, hippocampus, striatum and brainstem. It has been reported to show anticonvulsant properties in various models of animals and is mainly effective in the management of intractable epilepsy. It requires further research for the development of TSH analogues which are with good central nervous system permeability, high potency and metabolically more stable [58–60]. The neuropeptides, alterations and potential remedies in generalized epilepsy are enlisted in Table 2.
Table 2. Neuropeptides, alterations and potential remedies in generalized epilepsy
Таблица 2. Нейропептиды, изменения и потенциальные средства лечения генерализованной эпилепсии
|
Neuropeptide / |
Alterations and associations / Изменения и ассоциации |
Reference / Источник |
|
ACTH / АКТГ |
ACTH and cortisol amounts are decreased soon ahead of seizures, enhanced in course of seizures, and remain amplified following seizures / |
[61] |
|
Angiotensin / |
Bioactive peptides from the renin-angiotensin system, namely angiotensin II and IV, have reduced episodes of epilepsy. At increased levels, they reveal an antiepileptic action, enhance memory and learning / |
[62, 63] |
|
NPY / |
In seizures of epilepsy, there is an over expression of NPY, leading to the reduction of glutamate secretion. NPY1 receptors depict a postsynaptic response and are associated in neuroproliferative actions in the dentate gyrus. NPY2 receptors exhibit a presynaptic response and reduce the secretion of glutamate / |
[64] |
|
Proenkephalin / |
Proenkephalin depicts proconvulsant responses and is enhanced in a kainic acid-stimulated animal model of temporal epilepsy / |
[65] |
|
Somatostatin / |
Somatostatin is exhibited in dentate granule interneurons. It is expressed in seizures and reveals its action through the reduction of voltage gated Ca2+ channels / |
[66] |
|
Substance P / |
Substance P receptor-expressing cells of neurosurgically detached hippocampi in adults with epilepsy depict augmented dendritic arborisation / |
[67] |
|
TRH / ТРГ |
TRH might have an antiepileptic response in the management of intractable epilepsy and exhibits reduced proportions in generalized epilepsy / |
[68] |
|
VIP / ВИП |
VIP allays the intrathalamic rhythms revealed in epilepsy by stimulation of the VPAC2 receptor / |
[69] |
Note. ACTH – adrenocorticotropic hormone;
NPY – neuropeptide Y;
TRH – thyrotropin-releasing hormone;
VIP – vasoactive intestinal peptide.
Примечание. АКТГ – адренокортикотропный гормон;
ТРГ – тиреотропин-рилизинг-гормон;
ВИП – вазоактивный интестинальный пептид.
TREATMENT CASES OF EPILEPSY / ЛЕЧЕНИЕ ЭПИЛЕПСИИ
There are a number of other illustrations where unusual monogenic episodes of epilepsy have been examined thoroughly and management customized to the identified pathophysiological mechanism, with altering success.
- Most reliably efficient is the usage of ketogenic eating habit to commence cerebral energy metabolism away from glucose in people with deficiency of Glut-1, that might be noticeably successful. Control of seizures and higher quantity use of folic acid throughout pregnancy had enhanced over two decades. Regardless the alterartions in the AED use over period, the major congenital malformations (MCM) rates had remained unmodified perhaps owing to the continual usage of valproate, enhanced usage of clobazam and topiramate which are concerned with greater MCM rates and deficient of diminution in polytherapy [70].
- Controlled clinical trials in patients with medically intractable focal seizures managed with the RNS®System depict that closed-loop responsive neuroactivation to the seizure focus lessens the occurrence of disabling seizures, is well adpated, and is preferably secure.
- The findings from a computational technique which will ensure denser brain coverage in focal medically refractory epilepsy adults experiencing invasive checking by measuring neural actiion at “desired” missing electrode positions from signals of implanted electrodes. The tool contains an iterative algorithm that concurrently measures brain network models and the “missing” invasive EEG signals [71][72].
- US-based EAP registering kids and patients with treatment-resistant epilepsies, add-on cannabidiol efficiently decreased the median occurence of the chief motor and total seizures monthly following twelve weeks of management in the subcategory of patients with Lennox–Gastaut or Dravet syndrome [72][73].
- Though levetiracetam was not efficiently advanced to phenytoin, the findings, together with formerly described safety behavoir and relative ease of an administration, propose levetiracetam might be an suitable option to phenytoin as the first-option, second-line anticonvulsant drug in the cure of convulsive status epilepticus in paediatric [74].
There is a need to increase the quality of epilepsy research in India to understand the underlying biology of epilepsy which will further aid in devising new treatments and cure for chronic epilepsies. National and worldwide collaboration and financial support from funding organizations are needed to conduct quality research in India [75].
RECENT APPLICATIONS OF ANTIEPILEPTIC DRUGS / СОВРЕМЕННЫЕ ИССЛЕДОВАНИЯ ПРОТИВОЭПИЛЕПТИЧЕСКИХ ПРЕПАРАТОВ
The usage of AED molecules has become progressively more efficient through various factors: novel technologies that ensure the better identification of the seizure disease and its underlying ground; the formulation of innovative medicines and amplified information of older ones; and the extensive usage of AED proportion determination. The selection of a drug relies greatly on precise identification of seizure type, which may decide the action of the medicine. The recent applications of AEDs used to treat epilepsy are given in Table 3.
Table 3. Recent applications of antiepileptic drugs
Таблица 3. Современные исследования противоэпилептических препаратов
|
INN / МНН |
Drug form / |
Research stage / Стадия исследования |
Results / Results |
References / Источники |
|
Ezogabine / |
Nano-suspension / |
Zeta potential, saturation solubility, in vitro drug release, particle size / |
Enhance dissolution rate, increase saturation solubility, reduce particle size / |
[76][77] |
|
Lamotrigine / |
Nano-particles / |
In vitro drug release, drug content viscosity, pharmacokinetic study / |
Fast and pronounced absorption across the blood brain barrier by passing first pass metabolism / |
[78][79] |
|
Carbamazepine / |
Tablets (liquisolid compact) / |
Content uniformity, disintegration test, dissolution test / |
Improve dissolution rate / |
[80][81] |
|
Stiripentol / |
Tablets / |
Friability, disintegration time, in vitro release study / |
Improve patient compliance along with the rapid onset of action / |
[82][83] |
|
Levetiracetam / |
Extended release tablets / |
In vivo dissolution study, the drug release mechanism, stability study / |
Improve patient compliance / |
[84][85] |
|
Phenytoin sodium / |
Sustained release tablets / |
In vitro release study, content uniformity, friability / |
Increase solubility / |
[86][87] |
|
Diazepam / |
Orodispersible tablets / |
Uniformity of content, in vivo disintegration, dissolution / |
Good dissolution, appreciable buccal absorption, fast in vivo disintegration / |
[88][89] |
Note. INN – international nonproprietary name.
Примечание. МНН – международное непатентованное наименование.
CONCLUSION / ЗАКЛЮЧЕНИЕ
Epilepsy is a neurological disease typified by recurring incidents of unusual neural dynamics in the central nervous system. The specific clinical indications or impairments happened through such seizures rely on the specific brain part involve in the disease. By clear finding of the brain part involved, various biomarkers involved in the atypical electrical brain action new treatment strategies can be searched. The kind of remedy suggested will rely on various factors namely the occurence and harshness of the seizures as well as the age of person, overall health and record of the medical past.
References
1. López-Gómez M.L., Espinola M., Ramirez-Bermudez J., et al. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008; 4 (6): 1235–9. https://doi.org/10.2147/ndt.s3990.
2. Moshé S.L., Perucca E., Ryvlin P., Tomsan T. Epilepsy: new advances. Lancet. 2015; 385 (9971): 884–98. https://doi.org/10.1016/S01406736(14)60456-6.
3. Fisher R.S., Cross J.H., French J.A., et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58 (4): 522–30. https://doi.org/10.1111/epi.13670.
4. Dhiman P. Barbiturates in treatment of epilepsy. JDDT. 2013; 1 (8): 15–22.
5. Badawy R.A., Harvey A.S., Macdonald R.A. Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy – Part 1. J Clin Neurosci. 2009; 16 (3): 355–65. https://doi.org/10.1016/j.jocn.2008.08.026.
6. Singh S., Singh T.G., Rehni A.K., et al. Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion. 2021; 58: 213–26. https://doi.org/10.1016/j.mito.2021.03.009.
7. Werner F.M., Coveñas R. Classical neurotransmitters and neuropeptides involved in generalized epilepsy: a focus on antiepileptic drugs. Curr Med Chem. 2011; 18 (32): 4933–48. https://doi.org/10.2174/092986711797535191.
8. de Souza E.A., Salgado P.C. A psychosocial view of anxiety and depression in epilepsy. Epilepsy Behav. 2006; 8 (1): 232–8. https://doi.org/10.1016/j.yebeh.2005.10.011.
9. Hamid H., Ettinger A.B., Mula M. Anxiety symptoms in epilepsy: salient issues for future research. Epilepsy Behav. 2011; 22 (1): 63–8. https://doi.org/10.1016/j.yebeh.2011.04.064.
10. Hills M.D. The psychological and social impact of epilepsy. Neurology Asia. 2007; 12 (Suppl. 1): 10–2.
11. Mula M., Monaco F. Antiepileptic drugs and psychopathology of epilepsy: an update. Epileptic Disord. 2009; 11 (1): 1–9. https://doi.org/10.1684/epd.2009.0238.
12. Dupont S., Samson Y., Nguyen-Michel V.H., et al. Lateralizing value of semiology in medial temporal lobe epilepsy. Acta Neurol Scand. 2015; 132 (6): 401–9. https://doi.org/10.1111/ane.12409.
13. Barba C., Rheims S., Minotti L., et al. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain. 2016; 139 (Pt. 2): 444–51. https://doi.org/10.1093/brain/awv372.
14. Kaplan D.I., Isom L.L., Petrou S. Role of sodium channels in epilepsy. Cold Spring Harb Perspect Med. 2016; 6 (6): a022814. https://doi.org/10.1101/cshperspect.a022814.
15. Avanzini A., Franceschetti S., Mantegazza M. Epileptogenic channelopathies: experimental models of human pathologies. Epilepsia. 2007; 48 (Suppl. 2): 51–64. https://doi.org/10.1111/j.15281167.2007.01067.x.
16. Meldrum B.S., Rogawski M.A. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007; 4 (1): 18–61. https://doi.org/10.1016/j.nurt.2006.11.010.
17. White H.S., Smith M.D., Wilcox K.S. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007; 81: 85–110. https://doi.org/10.1016/S0074-7742(06)81006-8.
18. Kaminska B., Figiel I., Pyrzynska B., et al. Treatment of hippocampal neurons with cyclosporin A results in calcium overload and apoptosis which are independent on NMDA receptor activation. Br J Pharmacol. 2001; 133 (7): 997–1004. https://doi.org/10.1038/sj.bjp.0704177.
19. Amédée T., Robert A., Coles J.A. Potassium homeostasis and glial energy metabolism. Glia. 1997; 21 (1): 46–55.
20. Wen Y., Fu P., Wu K., et al. Inhibition of calcineurin A by FK506 suppresses seizures and reduces the expression of GluN2B in membrane fraction. Neurochem Res. 2017; 42 (8): 2154–66. https://doi.org/10.1007/s11064-017-2221-0.
21. van ’t Klooster M.A., Leijten F.S.S., Huiskamp G., et al. High frequency oscillations in the intra-operative ECoG to guide epilepsy surgery (‘The HFO Trial’): study protocol for a randomized controlled trial. Trials. 2015; 16: 422. https://doi.org/10.1186/s13063-015-0932-6.
22. Blumcke I., Spreafico R., Haaker G., et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017; 377: 1648–56. https://doi.org/10.1056/NEJMoa1703784.
23. Bozzi Y., Dunleavy M., and Henshall D.C. Cell signaling underlying epileptic behavior. Front Behav Neurosci. 2011; 5: 45. https://doi.org/10.3389/fnbeh.2011.00045.
24. Jacob T.C., Moss S.J., Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008; 9 (5): 331–43. https://doi.org/10.1038/nrn2370.
25. Hughes J.R. Gamma, fast, and ultrafast waves in the brain: their relationships with epilepsy and behavior. Epilepsy Behav. 2008; 13 (1): 25–31. https://doi.org/10.1016/j.yebeh.2008.01.011.
26. Chen J.W., Naylor D.E., Wasterlain C.G. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007; 186: 7–15.
27. Tsai M.L., Shen B., Leung L.S. Seizure induced by GABAB-receptor blockade in early-life induced long-term GABA(B) receptor hypofunction and kindling facilitation. Epilepsy Res. 2008; 79 (2–3): 187–200. https://doi.org/10.1016/j.eplepsyres.2008.02.001.
28. Bach Justesen A., Eskelund Johansen A.B., Martinussen N.I., et al. Added clinical value of the inferior temporal EEG electrode chain. Clin Neurophysiol. 2018; 129 (1): 291–5. https://doi.org/10.1016/j.clinph.2017.09.113.
29. Alvim M.K.M., Morita M.E., Yasuda C.L., et al. Is inpatient ictal videoelectroencephalographic monitoring mandatory in mesial temporal lobe epilepsy with unilateral hippocampal sclerosis? A prospective study. Epilepsia. 2018; 59 (2): 410–9. https://doi.org/10.1111/epi.13977.
30. Vakharia V.N., Sparks R., O'Keeffe A.G., et al. Accuracy of intracranial electrode placement for stereoencephalography: a systematic review and meta-analysis. Epilepsia. 2017; 58 (6): 921–32. https://doi.org/10.1111/epi.13713.
31. Kannan L., Vogrin S., Bailey C., et al. Centre of epileptogenic tubers generate and propagate seizures in tuberous sclerosis. Brain. 2016; 139 (Pt. 10): 2653–67. https://doi.org/10.1093/brain/aww192.
32. Chen C.M., Lin J.K., Liu S.H., Lin-Shiau S.Y. Novel regimen through combination of memantine and tea polyphenol for neuroprotection against brain excitoxicity. J Neurosci Res. 2008; 86 (12): 2696–704. https://doi.org/10.1002/jnr.21706.
33. Vincent P., Mulle C. Kainate receptors in epilepsy and excitotoxicity. Neuroscience. 2008; 158 (1): 309–23. https://doi.org/10.1016/j.neuroscience.2008.02.066.
34. Barker-Haliski M.B., White H.S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med. 2015; 5 (8): a022863. https://doi.org/10.1101/cshperspect.a022863.
35. Durand D., Carniglia L., Caruso C., Lasaga M. mGlu3 receptor and astrocytes: partners in neuroprotection. Neuropharmacology. 2013; 66: 1–11. https://doi.org/10.1016/j.neuropharm.2012.04.009.
36. Tang F.R., Chia S.C., Chen P.M., et al. Metabotropic glutamate receptor 2/3 in the hippocampus of patients with mesial temporal lobe epilepsy, and of rats and mice after pilocarpine-induced status epilepticus. Epilepsy Res. 2004; 59 (2–3): 167–80. https://doi.org/10.1016/j.eplepsyres.2004.04.002.
37. Borowicz K.K., Zarczuk R., Latalski M., Borowicz K.M. Reboxetine and its influence on the action of classical antiepileptic drugs in the mouse maximal electroshock model. Pharmacol Rep. 2014; 66 (3): 430–5. https://doi.org/10.1016/j.pharep.2013.11.009.
38. Veronesi M.C., Kubek D.J., Kubek M.J. Intranasal delivery of a thyrotropin-releasing hormone analog attenuates seizures in the amygdala-kindled rat. Epilepsia. 2007; 48 (12): 2280–6. https://doi.org/10.1111/j.1528-1167.2007.01218.x.
39. Hesdorffer D.C., Ishihara L., Mynepalli L., et al. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012; 72 (2): 184–91. https://doi.org/10.1002/ana.23601.
40. Epps S.A., Weinshenker D. Rhythm and blues: animal models of epilepsy and depression comorbidity. Biochem Pharmacol. 2013; 85 (2): 135–46. https://doi.org/10.1016/j.bcp.2012.08.016.
41. Hamid H., Kanner A.M. Should antidepressant drugs of the selective serotonin reuptake inhibitor family be tested as antiepileptic drugs? Epilepsy Behav. 2013; 26 (3): 261–5. https://doi.org/10.1016/j.yebeh.2012.10.009.
42. Bagdy G., Kecskemeti V., Riba P., Jakus R. Serotonin and epilepsy. J Neurochem. 2007; 100 (4): 857–73. https://doi.org/10.1111/j.14714159.2006.04277.x.
43. Jaseja H. Pedunculopontine nucleus stimulation: potent therapeutic role in intractable epilepsy. Epilepsy Behav. 2013; 27 (1): 280. https://doi.org/10.1016/j.yebeh.2013.01.021.
44. Acon-Chen C., Koenig A.J., Smith G.R., et al. Evaluation of acetylcholine, seizure activity and neuropathology following high-dose nerve agent exposure and delayed neuroprotective treatment drugs in freely moving rats. Toxicol Mech Methods. 2016; 26 (5): 378–88. https://doi.org/10.1080/15376516.2016.1197992.
45. Nemtsas P., Birot G., Pittau F., et. al. Source localization of ictal epileptic activity based on high-density scalp EEG data. Epilepsia. 2017; 58 (6): 1027–36. https://doi.org/10.1111/epi.13749.
46. Hoda J.C., Gu W., Friedli M., et al. Human nocturnal frontal lobe epilepsy: pharmacogenomic profiles of pathogenic nicotinic acetylcholine receptor beta-subunit mutations outside the ion channel pore. Mol Pharmacol. 2008; 74 (2): 379–91. https://doi.org/10.1124/mol.107.044545.
47. Kovac S., Walker M.C. Neuropeptides in epilepsy. Neuropeptides. 2013; 47 (6): 467–75. https://doi.org/10.1016/j.npep.2013.10.015.
48. van den Pol A.N. Neuropeptide transmission in brain circuits. Neuron. 2012; 76 (1): 98–115. https://doi.org/10.1016/j.neuron.2012.09.014.
49. Mytinger J.R., Joshi S. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol. 2012; 27 (10): 1289–94. https://doi.org/10.1177/0883073812455692.
50. Baram T.Z. Models for infantile spasms: an arduous journey to the Holy Grail. Ann Neurol. 2007; 61 (2): 89–91. https://doi.org/10.1002/ ana.21075.
51. Ramanujam B., Bharti K., Viswanathan V., et al. Can ictal-MEG obviate the need for phase II monitoring in people with drug-refractory epilepsy? A prospective observational study. Seizure. 2017; 45: 17–23. https://doi.org/10.1016/j.seizure.2016.10.013.
52. Jaseja H., Jaseja B., Badaya S., Tonpay P. Superior therapeutic efficacy of adrenocorticotrophic hormone (ACTH) in infantile spasms: emerging evidence. Epilepsy Behav. 2012; 25 (2): 250. https://doi.org/10.1016/j.yebeh.2012.08.003.
53. Koneru A., Satyanarayana S., Rizwan S. Endogenous opioids: their physiological role and receptors. Glob J Pharmacol. 2009; 3 (3): 149–53.
54. Park E.H., Madsen J.R. Granger causality analysis of interictal iEEG predicts seizure focus and ultimate resection. Neurosurgery. 2018; 82 (1): 99–109. https://doi.org/10.1093/neuros/nyx195.
55. Tomlinson S.B., Porter B.E., Marsh E.D. Interictal network synchrony and local heterogeneity predict epilepsy surgery outcome among pediatric patients. Epilepsia. 2017; 58 (3): 402–11. https://doi.org/10.1111/epi.13657.
56. Zweiphenning W.J., van 't Klooster M.A., van Diessen E., et al. High frequency oscillations and high frequency functional network characteristics in the intraoperative electrocorticogram in epilepsy. Neuroimage Clin. 2016; 12: 928–39. https://doi.org/10.1016/j. nicl.2016.09.014.
57. Tallent M.K., Qiu C. Somatostatin: anendogenous antiepileptic. Mol Cell Endocrinol. 2008; 286 (1–2): 96–103. https://doi.org/10.1016/j.mce.2007.12.004.
58. De Bundel D., Aourz N., Kiagiadaki F., et al. Hippocampal sst(1) receptors are autoreceptors and do not affect seizures in rats. Neuroreport. 2010; 21 (4): 254–8. https://doi.org/10.1097/WNR.0b013e3283353a64.
59. Choi Y.S., Lin S.L., Lee B., et al. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007; 27 (11): 2999–3009. https://doi.org/10.1523/JNEUROSCI.4913-06.2007.
60. Burns S.P., Santaniello S., Yaffe R.B., et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci U S A. 2014; 111 (49): E5321–30. https://doi.org/10.1073/pnas.1401752111.
61. Hebbink J., Meijer H., Huiskamp G., et al. Phenomenological network models: lessons for epilepsy surgery. Epilepsia. 2017; 58 (10): e147–51. https://doi.org/10.1111/epi.13861.
62. Centeno M., Tierney T.M., Perani S., et al. Combined electroencephalography–functional magnetic resonance imaging and electrical source imaging improves localization of pediatric focal epilepsy. Ann Neurol. 2017; 82 (2): 278–87. https://doi.org/10.1002/ana.25003.
63. Roehri N., Pizzo F., Lagarde S., et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol. 2018; 83 (1): 84–97. https://doi.org/10.1002/ana.25124.
64. Reilly M.T., Milner L.C., Shirley R.L., et al. 5-HT2C and GABAB receptors influence handling-induced convulsion severitiy in chromosome 4 congenic and DBA/2J background strain mice. Brain Res. 2008; 1198: 124–31. https://doi.org/10.1016/j.brainres.2008.01.024.
65. Khomane K.S., Meena C.L., Jain R., Bansal A.K. Novel thyrotropinreleasing hormone analogs: a patent review. Expert Opin Ther Pat. 2011; 21 (11): 1673–91. https://doi.org/10.1517/13543776.2011.623127.
66. Weiczner R., Krisztin-Péva B., Mihály A. Blockade of AMPA-receptors attenuates 4-aminopyridine seizures, decreases the activation of inhibitory neurons but is ineffective against seizure-related astrocytic swelling. Epilepsy Res. 2008; 78 (1): 22–32. https://doi.org/10.1016/j.eplepsyres.2007.10.004.
67. Werner F.M., Covenas R. Genetically and exogeneously induced neurotransmitter and neuropeptide alterations in generalized epilepsies in a multiple neurotransmitter system. Epilepsia. 2010; 51 (Suppl. 2): 32. https://doi.org/10.1111/j.1528-1167.2010.02595.x.
68. Mitsukawa K., Lu X., Bartfai T. Galanin receptors and drug targets. Exp Suppl. 2010; 102: 7–23. https://doi.org/10.1007/978-3-03460228-0_2.
69. Okanishi T., Akiyama T., Mayo E., et al. Magnetoencephalography spike sources interrelate the extensive epileptogenic zone of tuberous sclerosis complex. Epilepsy Res. 2016; 127: 302–10. https://doi.org/10.1016/j.eplepsyres.2016.09.007.
70. R.R. Keni, Jose M., Reshma A.S., et al. Anti-epileptic drug and folic acid usage during pregnancy, seizure and malformation outcomes: changes over two decades in the Kerala Registry of Epilepsy and Pregnancy. Epilepsy Res. 2020; 159: 106250. https://doi.org/10.1016/j.eplepsyres.2019.106250.
71. Skarpaas T.L., Jarosiewicz B., Morrell M.J. Brain-responsive neurostimulation for epilepsy (RNS® System). Epilepsy Res. 2019; 153: 68–70. https://doi.org/10.1016/j.eplepsyres.2019.02.003.
72. Stacey W., Kramer W., Gunnarsdottir K., et al. Emerging roles of network analysis for epilepsy. Epilepsy Res. 2020; 159: 106265. https://doi.org/10.1016/j.eplepsyres.2019.106255.
73. Laux L.C., Bebin E.M., Checketts D., et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox–Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019; 154: 13–20. https://doi.org/10.1016/j.eplepsyres.2019.03.015.
74. Lyttle M.D., Rainford N.E.A., Gamble C., et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019; 393 (10186): 2125–34. https://doi.org/10.1016/S01406736(19)30724-X.
75. Dixit A.B., Banerjee J., Chandra P.S., Tripathi M. Recent advances in epilepsy research in India. Neurol India. 2017; 65 (Suppl.): S83–92. https://doi.org/10.4103/neuroindia.NI_1070_16.
76. Harris J.A., Murphy J.A. Retigabine (ezogabine) as add-on therapy for partial-onset seizures: an update for clinicians. Ther Adv Chronic Dis. 2011; 2 (6): 371–76. https://doi.org//10.1177/2040622311421542.
77. Revathi S., Dhanaraju M.D. Optimization and characterization ezogabine-loaded nanosuspension for enhancement of bioavailability by “bottom-up” technology using 32 factorial design. JDDT. 2019; 9 (3): 227–37. https://doi.org/10.22270/jddt.v9i3.2860.
78. Ebrahimi H.A., Ebrahimi F. The effect of lamotrigine on epilepsy. Iran J Neurol. 2012; 11 (4): 162–3.
79. Raj R.A., Saju J. Formulation and evaluation of lamatrigine nanoparticle incorporated in situ gel for epilepsy. Int J Pharmacy Pharm Res. 2018; 13 (4): 1–17.
80. Tolou-Ghamari Z., Zare M., Habibabadi J.M., Najafi M.R. A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J Res Med Sci. 2013; 18 (Suppl. 1): S81–5.
81. Remeth J.D., Kailas K.M., Vishwajeet G., et al. Formulation and evaluation of carbamazepine liquisolid compacts using novel carriers. Indian J Pharm Edu. 2017; 51 (2): S69–78. https://doi.org/10.5530/ijper.51.2s.52.
82. Brigo F., Igwe S.C., Bragazzi N.L. Stiripentol add-on therapy for drugresistant focal epilepsy. Cochrane Database Syst Rev. 2022; 9 (9): CD009887. https://doi.org/10.1002/14651858.
83. Samar A.A. Development of promising fast dissolving tablet of stiripentol: a novel antiepileptic drug. J Pharm Res. 2014; 8 (6): 823–34.
84. Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008; 4 (3): 507–23. https://doi.org/10.2147/ndt.s2937.
85. Paliwal H., Goyal S., Rathore K.S., et al. Formulation and evaluation of levetiracetam extend release tablet. Int J Pharm Sci Rev Res. 2016; 41 (1): 260–6.
86. Iivanainen M., Savolainen H. Side effects of phenobarbital and phenytoin during long-term treatment of epilepsy. Acta Neurol Scand Suppl. 1983; 97: 49–67. https://doi.org/10.1111/j.1600-0404.1983.tb01535.x.
87. Madhavi N., Sudhakar B., Ravikanth P.V., et al. Formulation and evaluation of phenyotin sodium sustained release matrix tablet. J Bioequiv Bioavavilab. 2012; 4: 128–33. https://doi.org/10.4172/jbb.1000125.
88. Ochoa J.G., Kilgo W.A. The role of benzodiazepines in the treatment of epilepsy. Curr Treat Options Neurol. 2016; 18 (4): 18. https://doi.org/10.1007/s11940-016-0401-x.
89. Abed K.K., Hussein A.A., Ghareeb M.M., et al. Formulation and optimization of orodispersible tablet of diazepam. AAPS Pharm Sci Tech. 2010; 11 (1): 356–61. https://doi.org/10.1208/s12249-0109387-y.
About the Authors
M. DhallIndia
Manish Dhall – M. Pharm., PhD, Assistant Professor
Rohtak, Haryana 124001
R. Kadian
India
Renu Kadian – M. Pharm., PhD, Principle
Sultanpur, Farrukhnagar, Gurgaon, Haryana 122507
P. Sharma
India
Prerna Sharma – Associate Professor
Yamunanagar, Haryana 135001
A. Hooda
India
Anil Hooda – M. Pharm., Assistant Professor, Department of Pharmaceutical Education and Research, South Campus, Bhainswal Kalan
Khanpur Kalan, Sonepat, Haryana 131409
P. Kumar
India
Pushpander Kumar – M. Pharm., PhD, Assistant Professor (T), Department of Pharmaceutical Education and Research, South Campus, Bhainswal Kalan
Khanpur Kalan, Sonepat, Haryana 131409
P. Mudgal
India
Priya Mudgal – M. Pharm., Assistant Professor, Faculty of Pharmaceutical Sciences
Bahadurgarh, Haryana 124507
K. Singh
India
Kulwant Singh – M. Pharm., Assistant Professor
Sirsa, Haryana 125055
A. Arya
India
Khanpur Kalan, Sonepat, Haryana 131409
N. Rani
India
Nidhi Rani – M. Pharm., PhD, Associate Professor, Pharmaceutical Chemistry
Rajpura, Punjab 140401
Review
For citations:
Dhall M., Kadian R., Sharma P., Hooda A., Kumar P., Mudgal P., Singh K., Arya A., Rani N. Unravelling the neurochemical maze: neurotransmitters, neuropeptides and novel drug modes of action based on epilepsy pathophysiology. Epilepsy and paroxysmal conditions. 2023;15(3):282–293. https://doi.org/10.17749/2077-8333/epi.par.con.2023.152

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































