Scroll to:
Cognitive impairment in patients with juvenile myoclonic epilepsy
https://doi.org/10.17749/2077-8333/epi.par.con.2024.167
Abstract
Background. Сognitive impairment is one of the major epilepsy-related comorbidities. Upon long-term disease course, a decline in cognitive functions occurs in about 70–80% of cases. Juvenile myoclonic epilepsy (JME) is one of the most common forms of epilepsy (about 9.3%). Compared with other forms of idiopathic generalized epilepsy, JME is featured with high risk of seizures along with lowered patient compliance to treatment as well as a danger of developing drug resistance that may be a cause of cognitive disorder.
Objective: to review research publications on cognitive impairment in JME, discuss its putative causes, describe neuropsychological profile for JME patients.
Material and methods. The search was carried out in eLibrary, PubMed/MEDLINE, and Google Scholar databases using keywords and their combinations: “cognitive impairment”, “cognitive disorder”, “cognitive functions”, “neuropsychology”, “epilepsy”, “juvenile myoclonic epilepsy”, “JME”, “idiopathic generalized epilepsy”, “antiepileptic drugs”. We analyzed the articles published over the past 5 years and some earlier works of significant scientific interest. All articles were published in English or Russian languages.
Results. A total of 895 articles were found in databases. Comprehensive screening, evaluation of full-text articles eligibility in accordance with the criteria for selecting and deleting duplicates allowed to include 3 scientific publications in Russian and 67 scientific publications in English in the literature review. The main causes of cognitive impairment in JME patients were analyzed followed by describing relevant neuropsychological profile. Diagnostic tools and current opportunities for correction of cognitive disfunctions were considered as well.
Conclusion. The underlying causes of cognitive impairment in JME patients are multifactorial in nature and require further research. However, in this patient cohort prominent obstacles remain in identifying and timely correcting such disorders. Approving uniform diagnostic and therapeutic standards, developing rehabilitation methods for cognitive impairment in epilepsy will help improve the quality of life in JME patients.
Keywords
For citations:
Lysova K.D., Kuznetsov I.K., Paramonova A.I., Usoltseva A.A., Kantimirova E.A., Shnayder N.A., Dmitrenko D.V. Cognitive impairment in patients with juvenile myoclonic epilepsy. Epilepsy and paroxysmal conditions. 2024;16(1):77-87. https://doi.org/10.17749/2077-8333/epi.par.con.2024.167
INTRODUCTION / ВВЕДЕНИЕ
Cognitive impairment is one of the major comorbid conditions in epilepsy [1]. Upon long-term disease course, a decline in cognitive functions occurs in about 70–80% of cases [2][3].
Juvenile myoclonic epilepsy (JME) is one of the most common forms of epilepsy (about 9.3% of all forms) [4][5]. Compared to other forms of idiopathic generalized epilepsy, JME is featured with a high risk of seizures along with lowered patient compliance to treatment as well as a risk of developing drug-resistant disease [6][7] that may cause cognitive impairment.
JME is the most common syndrome of genetic generalized epilepsies (GGE) with onset in adolescence and adulthood [8]. The disease prevalence varies from one to three cases per 10 thousand people [9]. JME is characterized by a triad of seizures: myoclonic, generalized tonic-clonic seizures (GTCS), and absence of seizures. Myoclonic seizures usually occur upon awakening and during fatigue and are a mandatory criterion for JME diagnostics. GTCS are found in more than 90% of patients, absence of seizures occurs in 1/3 of JME patients [10–12]. Generalized spike-wave and polyspike-wave activity with electroencephalogram (EEG)-monitored 3–5.5 Hz frequency is a typical sign for JME [8].
In most cases, despite the benign course of the disease, long-term antiepileptic drugs (AEDs) use and lifelong exclusion of disease provoking factors are required [7].
Recent studies indicate declines and specific impairment in cognitive functions, but it is not well understood whether such alterations are specific solely to JME patients or common for all GGE subtypes [13].
In addition, the problem of a unified taxonomy for determining cognitive impairment in patients with epilepsy has not been resolved. The lack of uniform diagnostic criteria poses prominent obstacles in clinical practice and investigation of epilepsy [1].
Among brain activities most complex are cognitive functions such as memory, gnosis, speech, praxis and intelligence [14], through which the process of rational cognition of the world is carried out (Fig. 1).
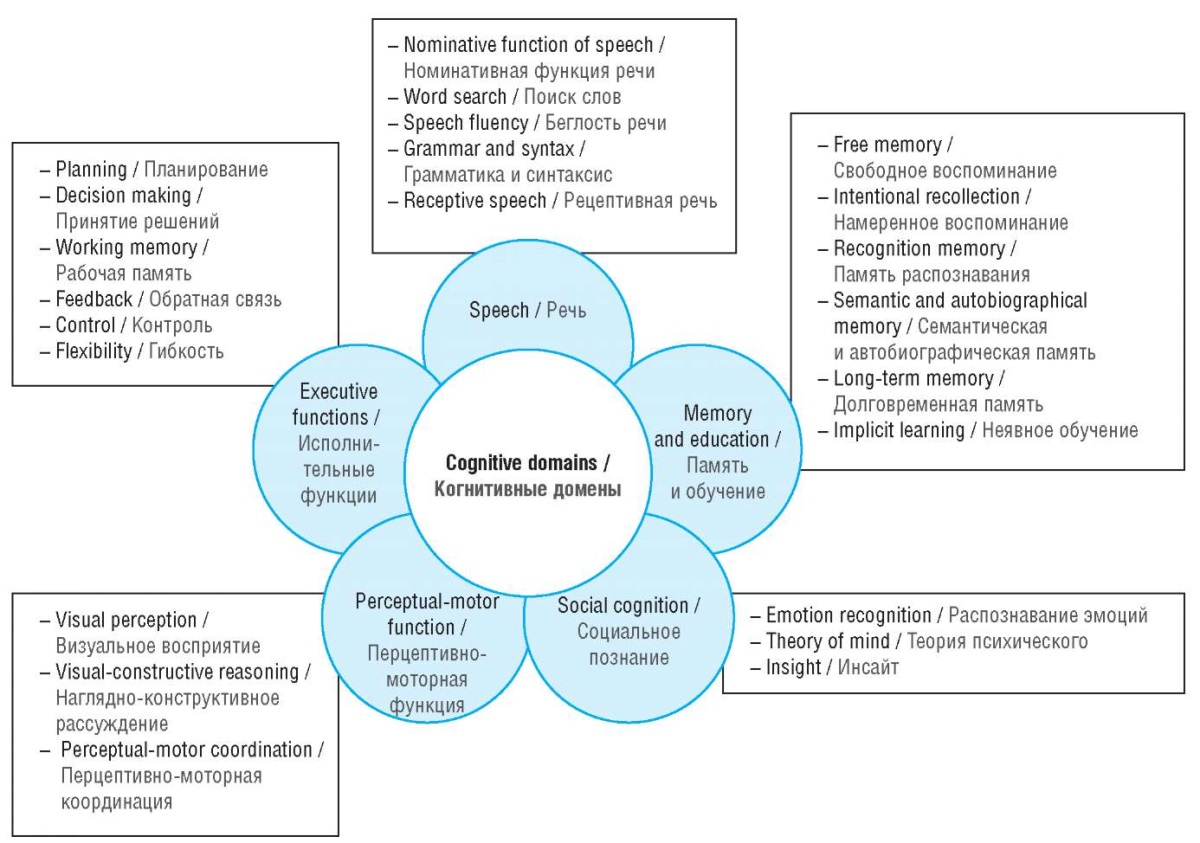
Figure 1. Human cognitive domains (adapted from [1])
Рисунок 1. Когнитивные домены человека (адаптировано из [1])
A selective cognitive impairment in JME patients is observed in most cases [15][16]. Moreover, there is a high risk of developing mental disorders such as anxiety, depression, and impulsivity, which can lead to impaired socialization and psychological problems [17][18] able to precede the onset of epileptic seizures [17].
Objective: to review research publications on cognitive impairment in JME, discuss its putative causes, describe neuropsychological profile for JME patients.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
The search for publications was carried out in the databases eLibrary, PubMed/MEDLINE, and Google Scholar, using keywords and their combinations in English and Russian: “cognitive impairment”, “cognitive disorders”, “cognitive functions”, “neuropsychology”, “juvenile myoclonic epilepsy”, “JME”, “idiopathic genetic epilepsy”, “anti-epileptic drugs”. We analyzed articles published over the past five years and earlier works referred to therein. Articles published in English and Russian that included studies with JME patients and cognitive impairment were selected for analysis.
A total of 895 articles were found in the databases. After carefully screening, assessing full-text article eligibility in accordance with the selection criteria and removing duplicate texts, 3 research publications in Russian and 67 research publications in English were included in the literature review.
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Сauses of cognitive impairment in epilepsy / Причины когнитивных нарушений при эпилепсии
It is believed that a multifactorial cognitive impairment develops in epilepsy. Available publications demonstrate that three major factors influencing the development of cognitive impairment are involved: the etiology of epilepsy [13], epileptic seizures and EEG-verified epileptiform activity as well as adverse events due to AEDs [19–21]. Depending on which factor has a greater impact on cognitive functions, the timing, degree, and course of cognitive impairment may vary [22], whereas ictal and postictal cognitive dysfunction are reversible that has been extensively discussed [23].
It is generally accepted that a progressive decline in cognitive functions occurs in long-term and drug-resistant epilepsy with the accumulation of epileptic seizures throughout life. However, epilepsy and its accompanying conditions such as depression and memory loss, can, but should not, be interrelated [24]. The term “comorbidity” does not imply or exclude a causal relationship between cognitive impairment and epilepsy [25].
Structural brain disorders and genetic predisposition / Структурные нарушения головного мозга и генетическая предрасположенность
Theoretically, cognitive impairment in JME patients may result from microstructural changes in brain gray and white matter [26].
In GGE, much attention is paid to neurogenetic etiology, but research in this field remains sparce [27]. To date, only six genes bearing pathogenic variants (GABRA1, GABRD, EFHC1, BRD2, CASR and ICK) with Mendelian and complex inheritance are considered as the major JME susceptibility alleles and can determine development of microstructural changes in the brain [26].
Despite that the definition of JME proposed by the International League Against Epilepsy (ILAE) in 2022 [8] points at the lack of magnetic resonance imaging (MRI) structural changes, histopathological, morphometric studies reveal microstructural changes in both gray and white matter of the brain in this patient group [28]. MRI data show that changes in brain white matter and reduced thalamus volume were found in newly JME-diagnosed patients [29][30]. Microstructural changes have also been described in the prefrontal cortex and thalamus [31] as well as hippocampal regions [32][33].
In the 2018 meta-analysis conducted by the ENIGMA (The Enhancing Neuro Imaging Genetics through Meta-Analysis) consortium, altered thickness of the precentral gyri was identified in 367 GGE patients compared to control group [34], which is consistent with some data from a more recent JME meta-analysis.
Meta-analysis by D. Kazis et al. (2021) assessing 12 studies and 365 patients used voxel-based morphometry to identify structural changes such as increased gray matter level in the left middle cingulate/paracingulate gyrus, right superior frontal gyrus, left precentral gyrus, right supplementary motor area, and left supplementary gyrus of the motor area. A decreased volume of gray matter was also detected in the left thalamus and the left insula of the brain [35].
Hippocampal neuroimaging and spectroscopy data collected by L. Caciagli et al. (2019) identified that 50% JME patients and paired siblings had structural changes in hippocampal shape as well as altered left hippocampus. While assessing mesotemporal functions using neuropsychological tests and functional MRI, no overt impairment in verbal and nonverbal memory was detected, but altered rate of activation for mesotemporal functions was revealed. In addition, patients with hippocampal malrotation showed lowered frontal cortex activation during verbal memory and more prominently involved left posterior hippocampus during visual memory activation [32].
Moreover, neuroimaging data assessed in a series of studies with newly diagnosed JME patients showed structural changes in the brain, including a less modular cortical network as well as larger volume and thickness of the fronto-parietal-temporal region [36–38]. Altered signal was detected in the cortico-cortical, orbitofrontal, ventrolateral frontal, premotor and temporopolar regions. The latter was also demonstrated to be most affected by abnormal embedding of cognitive frontoparietal, dorsal and limbic networks [39].
The study by D.A. Lee et al. (2021) uncovered changes in the local and global networks of the hippocampus in 35 newly diagnosed JME patients, which may also result in developing specific cognitive impairment [40].
Thus, study data show that JME patients more commonly have: altered volume, structure and morphological hippocampus properties [32][40], affected structure in the prefrontal and cingulate cortex (such as increased complexity of folding and altered surface area) [39] and changes in motor cortex hyperactivation based on functional MRI data [41]. Despite this, the specificity of frontotemporal endophenotypic properties in JME patients requires to be further investigated [38].
Epileptic seizures and epileptiform activity on electroencephalogram / Эпилептические приступы и наличие эпилептиформной активности на электроэнцефалограмме
Seizure frequency and epileptiform EEG activity in JME patients may also cause persistent and progressive cognitive impairment. In particular, the study by Z.E. Balcik et al. (2020) showed dysfunction of the frontal lobes in 60 JME patients, and those with one-second-longer generalized activity performed worse tests assessing attention and had more errors in perseveration. However, no significant correlation between focal EEG patterns and test scoring for frontal lobe cognitive functions was found in JME group [42].
Study by S. Sezikli et al. (2018) on JME with EEG-based 15 asynchronous vs. 15 synchronous generalized activity patients revealed a more prominent decline in frontal lobe cognitive functions [15].
In epilepsy, transient cognitive impairment (TCI) is commonly associated with generalized activity [43]. In addition, up to 50% of patients with epileptiform activity are found to have TCI upon cognitive assessment [44] suggesting that epileptiform changes may affect cognition in epilepsy or be a marker of more severe disease associated with cognitive impairment. Thus, it has been shown that deterioration of memory and cognitive functions is associated with longer cumulative duration of epileptiform activity based on 24-hour EEG monitoring in GGE patients [45]. Moreover, it also found that difference in IQ scores was more likely to be accounted for by total duration of sleep vs. wakefulness epileptiform discharge [45]. In addition, changes in long-term memory functions were accounted for by longer total duration of wakefulness vs. sleep epileptiform activity [46].
An earlier retrospective study showed that decreased mean duration of generalized seizures, fewer epileptiform discharge and lower EEG spike density were associated with longer seizure free period in GGE patients [47].
Cognitive impairment is recorded mainly outside remission and depends on the nature of epileptic seizures. Myoclonic seizures along with GTCS vs. isolated myoclonic seizures more often resulted in developing cognitive impairment [17].
S.C. Tromp et al. (2003) showd that in children EEG-monitored epileptiform activity during cognitive testing caused no prominently decreased IQ scores, but significantly reduced the reaction time to binary choice, i.e. the speed of information processing. The frequency of epileptic seizures and the disease severity influenced the decline in stable aspects of cognitive functions resulting in lower IQ scores [48].
Epileptiform discharge may be considered a useful biomarker for response to AED treatment, because it was evidenced that they correlate with seizure control and cognitive epilepsy outcomes [46].
The study by A.S. Eriksson et al. (2001) suggested that improvement in mental speed and behavior following changes in antiepileptic therapy is related to lowered interictal epileptiform activity [49].
Effect of antiepileptic drugs on cognitive functions / Влияние противоэпилептических препаратов на когнитивные функции
In most cases, patients with epilepsy should receive long-term antiepileptic treatment, which can impact on cognitive functions, both positively and negatively. The number of medications taken, poor adherence to drug treatment and AED-related side effects affect the patients' quality of life [50][51].
R. Moavero et al. (2017) identified several factors involved in emerging cognitive side effects in epilepsy therapy. Among them are polytherapy, introduction of same-mechanism-of-action AEDs, and improper drug dosing [52].
According to M. Mula et al. (2009), older-generation AEDs such as phenobarbital, phenytoin, carbamazepine and valproic acid affect general cognitive indicators [53]. JME patients taking valproic acid monotherapy scored significantly lower than those matched for age, education, and gender on neuropsychological measures of attention, immediate verbal memory, mental flexibility, inhibitory control, working memory, processing speed, verbal memory delay, visual memory delay, naming and verbal fluency compared to the control group. The duration of epilepsy correlated with a decline in cognitive functions; in addition, patients with higher education showed less progression of cognitive deficits [54].
The effect of valproic acid on lowered cognitive functions in children born to mothers who took valproic acid during pregnancy has been proven. Compared to other AEDs such as lamotrigine, phenytoin and carbamazepine, decreased cognitive functions were recorded in the form of delayed speech development, lower IQ scores in 30–40% of valproic acid-treated patients [55]. A dose-dependent effect was also proven; when a mother took valproic acid at a dose of 1000 mg/day or higher, lower IQ scores, decreased verbal and non-verbal intelligence, decreased memory and impaired executive functions were recorded in paired children [56].
Higher AED doses and polytherapy are associated with aggravated overall side effects [57]. It has also been shown that each additional drug in treatment regimen can result in lowered objective cognitive parameters [58][59], which is in line with the results by L. Feldman et al. (2018) who suggested that after severity level of depressive symptoms the number of AEDs represents the second predictor for subjective cognitive impairment [60].
Of the AED-impaired cognitive domains, the most common reported AED are shown to affect attention, vigilance, and psychomotor speed [61]. In addition, the study by R.J. Quon et al. (2020) found that patients receiving AED polytherapy vs. monotherapy passed less efficiently tests on subjective cognition, verbal and working memory as well as information processing speed [61].
In adult patients, topiramate affected verbal functions primarily acting on verbal fluency [53][62]. F.M.C. Besag and M.J. Vasey (2021) uncovered that topiramate and phenobarbital in children specifically strongly elicited cognitive impairment, whereas topiramate alone was associated with speech impairment [63]. Lamotrigine and levetiracetam less likely cause cognitive impairment and in some patients may improve cognitive functions presumably by achieving seizure control [63].
Neuropsychological profile of JME patient / Нейропсихологический профиль пациента с ЮМЭ
It was previously thought that cognitive decline in patients with JME was relatively less common than in other forms of epilepsy. However, recent studies show a high percentage of selective cognitive and behavioral impairment [64]. In addition, there is evidence of the absence of cognitive impairment at the onset of the disease, before the start of taking AEDs, but such studies are few in number [65].
It is assumed that general cognitive abilities are negatively correlated with disease duration [13].
R.V. Magzhanov et al. (2017) analyzed the prevalence of cognitive impairment in 44 JME patients. A decline in Montreal scale-assessed cognitive functions was detected in 40% of patients. In addition, 33.3% of them showed subclinical manifestations of anxiety according to the Hospital Anxiety and Depression Scale (HADS) [66].
The study by D.N. Almane et al. (2019) with 111 JME children aged 8 to 18 years (41 patients with newly or recently diagnosed JME and 70 first-degree relatives) vs. control subjects showed an overall decline in cognitive functions. They also had a higher incidence of attention deficit hyperactivity disorder, depression, and anxiety [17].
Many studies have described dysregulatory disorders, including impaired speed of thinking, difficulty concentrating, difficulty switching, and difficulty making decisions. Impaired executive functions occur in JME patients experiencing difficulties planning tasks, forming goals and subsequent control [13][67]. Apart from this, a decline in phonemic and semantic speech fluency they suffer is also noted [13][68].
Meta-analysis by A. Loughman et al. (2017) evidenced about consistent decline in semantic knowledge and problem-solving skills, which replicates findings in mixed groups of GGE patients. However, intellectual abilities stay within normal range, although slightly lower than in control groups. In addition, disturbances in visuospatial perception and visual attention are noted [69].
The involvement of temporal and hippocampal process-dependent cognitive functions in JME patients requires to be further explored. Several studies reported a normal performance in learning and memory tests [68], whereas others detailed deficits in short- and long-term memory compared to control subjects [70]. Memory impairment was considered to be a consequence of insufficient visual and verbal learning [15]. Conflicting data may be partly due to JME syndromic heterogeneity [28][54].
CONCLUSION / ЗАКЛЮЧЕНИЕ
The causes underlying cognitive impairment in JME patients are multifactorial and require further assessment. Identifying the mechanisms of developing cognitive impairment will allow to promptly prevent such conditions.
The cognitive profile of JME patients is represented by mean general level of intelligence, which is accompanied by impaired semantic and phonemic fluency, working memory and a wide range of executive functions, varying from mild to severe level.
At the moment, significant obstacles remain in identifying and timely correcting cognitive impairment in JME patients. Approving uniform diagnostic and therapeutic standards and developing rehabilitation methods for cognitive disorders in patients with epilepsy will help improve the quality of life for this patient cohort.
References
1. Norman M., Wilson S.J., Baxendale S., et al. Addressing neuropsychological diagnostics in adults with epilepsy: introducing the international classification of cognitive disorders in epilepsy: The IC CODE Initiative. Epilepsia Open. 2021; 6 (2): 266–75. https://doi.org/10.1002/epi4.12478.
2. Helmstaedter C., Witt J.A. Clinical neuropsychology in epilepsy: theoretical and practicalissues. Handb Clin Neurol. 2012; 107: 437–59. https://doi.org/10.1016/B978-0-444-52898-8.00036-7.
3. Anthony J.C., Eaton W.W., Henderson A.S. Looking to the future in psychiatric epidemiology. Epidemiol Rev. 1995; 17 (1): 240–2. https://doi.org/10.1093/oxfordjournals.epirev.a036182.
4. Juul-Jensen P., Foldspang A. Natural history of epileptic seizures. Epilepsia. 1983; 24 (3): 297–312. https://doi.org/10.1111/j.1528-1157.1983.tb04893.x.
5. Syvertsen M., Nakken K.O., Edland A., et al. Prevalence and etiology of epilepsy in a Norwegian county – a population based study. Epilepsia. 2015; 56 (5): 699–706. https://doi.org/10.1111/epi.12972.
6. Mukhin K.Yu., Freidkova N.V., Glukhova L.Yu., et al. Juvenile myoclonic epilepsy: a focus on the efficacy of therapy and the rate of relapses according to long-term follow-up data. Russian Journal of Child Neurology. 2015; 10 (4): 7–16 (in Russ.). https://doi.org/10.17650/2073-8803-2015-10-4-7-16.
7. Stevelink R., Koeleman B.P., Sander J.W., et al. Refractory juvenile myoclonic epilepsy: a meta-analysis of prevalence and risk factors. Eur J Neurol. 2019; 26 (6): 856–64. https://doi.org/10.1111/ene.13811.
8. Hirsch E., French J., Scheffer I.E., et al. ILAE definition of the idiopathic generalizedepilepsy syndromes: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022; 63 (6): 1475–99. https://doi.org/10.1111/epi.17236.
9. Syvertsen M., Hellum M.K., Hansen G., et al. Prevalence of juvenile myoclonic epilepsy in people <30 years of age – a populationbased study in Norway. Epilepsia. 2017; 58 (1): 105–12. https://doi.org/10.1111/epi.13613.
10. Elmali A.D., Auvin S., Bast T., et al. How to diagnose and classify idiopathic (genetic) generalized epilepsies. Epileptic Disord. 2020; 22 (4): 399–420. https://doi.org/10.1684/epd.2020.1192.
11. Yacubian E.M. Juvenile myoclonic epilepsy: challenges on its 60th anniversary. Seizure. 2017; 44: 48–52. https://doi.org/10.1016/j.seizure.2016.09.005.
12. Panayiotopoulos C.P., Obeid T., Waheed G. Absences in juvenile myoclonic epilepsy: a clinical and video-electroencephalographic study. Ann Neurol. 1989; 25 (4): 391–7. https://doi.org/10.1002/ana.410250411.
13. Ratcliffe C., Wandschneider B., Baxendale S., et al. Cognitive function in genetic generalized epilepsies: insights from neuropsychology and neuroimaging. Front Neurol. 2020; 11: 144. https://doi.org/10.3389/fneur.2020.00144.
14. Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. Neuropsychology assessment. 5th ed. Oxford University Press; 2012: 1200 pp.
15. Sezikli S., Pulat T.A., Tekin B., et al. Frontal lobe cognitive functions and electroencephalographic features in juvenile myoclonic epilepsy. Epilepsy Behav. 2018; 86: 102–7. https://doi.org/10.1016/j.yebeh.2018.06.009.
16. Chawla T., Chaudhry N., Puri V. Cognitive dysfunction in juvenile myoclonic epilepsy (JME) – a tertiary care center study. Ann Indian Acad Neurol. 2021; 24 (1): 40–50. https://doi.org/10.4103/aian.AIAN_663_19.
17. Almane D.N., Jones J.E., McMillan T., et al. The timing, nature, and range of neurobehavioral comorbidities in juvenile myoclonic epilepsy. Pediatr Neurol. 2019; 101: 47–52. https://doi.org/10.1016/j.pediatrneurol.2019.03.011.
18. Syvertsen M., Selmer K., Enger U., et al. Psychosocial complications in juvenile myoclonic epilepsy. Epilepsy Behav. 2019; 90: 122–8. https://doi.org/10.1016/j.yebeh.2018.11.022.
19. Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004; 3 (11): 663–72. https://doi.org/10.1016/S1474-4422(04)00906-8.
20. Helmstaedter C.A. Prediction of memory reserve capacity. Adv Neurol. 1999; 81: 271–9.
21. Shilkina O.S., Artyukhov I.P., Moskaleva P.B., et al. Cognitive disorders in juvenile myoclonic epilepsy. Int J Biomed. 2017; 7 (1): 9–14. https://doi.org/10.21103/Article7(1)_RA1.
22. Kim E.H., Ko T.S. Cognitive impairment in childhood onset epilepsy: up-to-date information about its causes. Korean J Pediatr. 2016; 59 (4): 155–64. https://doi.org/10.3345/kjp.2016.59.4.155.
23. Helmstaedter C., Elger C.E., Lendt M. Postictal courses of cognitive deficits in focal epilepsies. Epilepsia. 1994; 35 (5): 1073–8. https://doi.org/10.1111/j.1528-1157.1994.tb02557.x.
24. Keezer M.R., Sander J.W. Comorbidity as an epidemiological construct. Lancet Neurol. 2016; 15 (1): 32. https://doi.org/10.1016/S1474-4422(15)00352-X.
25. Helmstaedter C., Witt J.A. Epilepsy and cognition – a bidirectional relationship? Seizure. 2017; 49: 83–9. https://doi.org/10.1016/j.seizure.2017.02.017.
26. Gilsoul M., Grisar T., Delgado-Escueta A.V., et al. Subtle brain developmental abnormalities in the pathogenesis of juvenile myoclonic epilepsy. Front Cell Neurosci. 2019; 13: 433. https://doi.org/10.3389/fncel.2019.00433.
27. Pulsipher D.T., Dabbs K., Tuchsherer V., et al. Thalamofrontal neurodevelopment in new-onset pediatric idiopathic generalized epilepsy. Neurology. 2011; 76 (1): 28–33. https://doi.org/10.1212/WNL.0b013e318203e8f3.
28. de Araújo Filho G.M., Jackowski A.P., Lin K., et al. Personality traits related to juvenile myoclonic epilepsy: MRI reveals prefrontal abnormalities through a voxel-based morphometry study. Epilepsy Behav. 2009; 15 (2): 202–7. https://doi.org/10.1016/j.yebeh.2009.03.011.
29. Ekmekci B., Bulut H.T., Gümüştaş F., et al. The relationship between white matter abnormalities and cognitive functions in new-onset juvenile myoclonic epilepsy. Epilepsy Behav. 2016; 62: 166–70. https://doi.org/10.1016/j.yebeh.2016.07.015.
30. Perani S., Tierney T.M., Centeno M., et al. Thalamic volume reduction in drug-naive patients with new-onset genetic generalized epilepsy. Epilepsia. 2018; 59 (1): 226–34. https://doi.org/10.1111/epi.13955.
31. Kim J.H. Grey and white matter alterations in juvenile myoclonic epilepsy: a comprehensive review. J Epilepsy Res. 2017; 7 (2): 77–88. https://doi.org/10.14581/jer.17013.
32. Caciagli L., Wandschneider B., Xiao F., et al. Abnormal hippocampal structure and function in juvenile myoclonic epilepsy and unaffected siblings. Brain. 2019; 142 (9): 2670–87. https://doi.org/10.1093/brain/awz215.
33. Lin K., de Araujo Filho G.M., Pascalicchio T.F., et al. Hippocampal atrophy and memory dysfunction in patients with juvenile myoclonic epilepsy. Epilepsy Behav. 2013; 29 (1): 247–51. https://doi.org/10.1016/j.yebeh.2013.06.034.
34. Whelan C.D., Altmann A., Botía J.A., et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018; 141 (2): 391–408. https://doi.org/10.1093/brain/awx341.
35. Kazis D., Petridis F., Chatzikonstantinou S., et al. Gray matter changes in juvenile myoclonic epilepsy. A voxel-wise meta-analysis. Medicina (Kaunas). 2021; 57 (11): 1136. https://doi.org/10.3390/medicina57111136.
36. Lin J.J., Dabbs K., Riley J.D., et al. Neurodevelopment in new-onset juvenile myoclonic epilepsy over the first 2 years. Ann Neurol. 2014; 76 (5): 660–8. https://doi.org/10.1002/ana.24240.
37. Garcia-Ramos C., Dabbs K., Lin J.J., et al. Progressive dissociation of cortical and subcortical network development in children with newonset juvenile myoclonic epilepsy. Epilepsia. 2018; 59 (11): 2086–95. https://doi.org/10.1111/epi.14560.
38. Wang G., Wu W., Xu Y., et al. Imaging genetics in epilepsy: current knowledge and new perspectives. Front Mol Neurosci. 2022; 15: 891621. https://doi.org/10.3389/fnmol.2022.891621.
39. Wandschneider B., Hong S.J., Bernhardt B.C., et al. Developmental MRI markers cosegregate juvenile patients with myoclonic epilepsy and their healthy siblings. Neurology. 2019; 93 (13): e1272–80. https://doi.org/10.1212/WNL.0000000000008173.
40. Lee D.A., Ko J., Lee H.J., et al. Alterations of the intrinsic amygdalahippocampal network in juvenile myoclonic epilepsy. Brain Behav. 2021; 11 (8): e2274. https://doi.org/10.1002/brb3.2274.
41. Caciagli L., Wandschneider B., Centeno M., et al. Motor hyperactivation during cognitive tasks: an endophenotype of juvenile myoclonic epilepsy. Epilepsia. 2020; 61 (7): 1438–52. https://doi.org/10.1111/epi.16575.
42. Balcik Z.E., Senadim S., Tekin B., et al. Do interictal EEG findings reflect cognitive function in juvenile myoclonic epilepsy? Epilepsy Behav. 2020; 111: 107281. https://doi.org/10.1016/j.yebeh.2020.107281.
43. Aarts J.H., Binnie C.D., Smit A.M., et al. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. 1984; 107 (1): 293–308. https://doi.org/10.1093/brain/107.1.293.
44. Binnie C.D. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003; 2 (12): 725–30. https://doi.org/10.1016/s1474-4422(03)00584-2.
45. Loughman A., Seneviratne U., Bowden S.C., et al. Epilepsy beyond seizures: predicting enduring cognitive dysfunction in genetic generalized epilepsies. Epilepsy Behav. 2016; 62: 297–303. https://doi.org/10.1016/j.yebeh.2016.07.010.
46. Gunawan C., Seneviratne U., D'Souza W. The effect of antiepileptic drugs on epileptiform discharges in genetic generalized epilepsy: a systematic review. Epilepsy Behav. 2019; 96: 175–82. https://doi.org/10.1016/j.yebeh.2019.04.030.
47. Wirrell E.C., Camfield C.S., Camfield P.R., et al. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med. 1997; 151 (2): 152–8. https://doi.org/10.1001/archpedi.1997.02170390042008.
48. Tromp S.C., Weber J.W., Aldenkamp A.P., et al. Relative influence of epileptic seizures and of epilepsy syndrome on cognitive function. J Child Neurol. 2003; 18 (6): 407–12. https://doi.org/10.1177/08830738030180060501.
49. Eriksson A.S., Knutsson E., Nergårdh A. The effect of lamotrigine on epileptiform discharges in young patients with drug-resistant epilepsy. Epilepsia. 2001; 42 (2): 230–6. https://doi.org/10.1046/j.1528-1157.2001.37799.x.
50. Nagabushana D., S P.K., Agadi J.B. Impact of epilepsy and antiepileptic drugs on health and quality of life in Indian children. Epilepsy Behav. 2019; 93: 43–8. https://doi.org/10.1016/j.yebeh.2019.01.021.
51. Nasyrova R.F., Sivakova N.A., Lipatova L.V., et al. Biological markers of the antiepileptic drugs efficacy and safety: pharmacogenetics and pharmacokinetics. Siberian Medical Review. 2017; 1: 17–25 (in Russ.). https://doi.org/10.20333/2500136-2017-1-17-25.
52. Moavero R., Santarone M.E., Galasso C., Curatolo P. Cognitive and behavioral effects of new antiepileptic drugs in pediatric epilepsy. Brain Dev. 2017; 39 (6): 464–9. https://doi.org/10.1016/j.braindev.2017.01.006.
53. Mula M., Trimble M.R. Antiepileptic drug-induced cognitive adverse effects: potential mechanisms and contributing factors. CNS Drugs. 2009; 23 (2): 121–37. https://doi.org/10.2165/00023210-200923020-00003.
54. Pascalicchio T.F., de Araujo Filho G.M., da Silva Noffs M.H., et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007; 10 (2): 263–7. https://doi.org/10.1016/j.yebeh.2006.11.012.
55. Li Y., Meador K.J. Epilepsy and pregnancy. Continuum. 2022; 28 (1): 34–54. https://doi.org/10.1212/CON.0000000000001056.
56. Craig J.J., Scott S., Leach J.P. Epilepsy and pregnancy: identifying risks. Pract Neurol. 2022; 22 (2): 98–106. https://doi.org/10.1136/practneurol-2019-002304.
57. Eddy C.M., Rickards H.E., Cavanna A.E. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011; 4 (6): 385–407. https://doi.org/10.1177/1756285611417920.
58. Witt J.A., Elger C.E., Helmstaedter C. Adverse cognitive effects of antiepileptic pharmacotherapy: each additional drug matters. Eur Neuropsychopharmacol. 2015; 25 (11): 1954–9. https://doi.org/10.1016/j.euroneuro.2015.07.027.
59. Witt J.A., Helmstaedter C. How can we overcome neuropsychological adverse effects of antiepileptic drugs? Expert Opin Pharmacother. 2017; 18 (6): 551–4. https://doi.org/10.1080/14656566.2017.1309025.
60. Feldman L., Lapin B., Busch R.M., Bautista J.F. Evaluating subjective cognitive impairment in the adult epilepsy clinic: effects of depression, number of antiepileptic medications, and seizure frequency. Epilepsy Behav. 2018; 81: 18–24. https://doi.org/10.1016/j.yebeh.2017.10.011.
61. Quon R.J., Mazanec M.T., Schmidt S.S., et al. Antiepileptic drug effects on subjective and objective cognition. Epilepsy Behav. 2020; 104 (Pt. A): 106906. https://doi.org/10.1016/j.yebeh.2020.106906.
62. Mula M. Topiramate and cognitive impairment: evidence and clinical implications. Ther Adv Drug Saf. 2012; 3 (6): 279–89. https://doi.org/10.1177/2042098612455357.
63. Besag F.M.C., Vasey M.J. Neurocognitive effects of antiseizure medications in children and adolescents with epilepsy. Paediatr Drugs. 2021; 23 (3): 253–86. https://doi.org/10.1007/s40272-021-00448-0.
64. Abarrategui B., Parejo-Carbonell B., García García M.E., et al. The cognitive phenotype of idiopathic generalized epilepsy. Epilepsy Behav. 2018; 89: 99–104. https://doi.org/10.1016/j.yebeh.2018.10.007.
65. Raatikainen M., Kälviäinen R., Jutila L., Äikiä M. Cognitive functioning in new-onset juvenile myoclonic epilepsy. Epilepsy Behav. 2020; 106: 107015. https://doi.org/10.1016/j.yebeh.2020.107015.
66. Magzhanov R.V., Anisimova D.V., Vlasov P.N., et al. Cognitive and emotional changes in patients with juvenile myoclonic epilepsy. Neurology, Neuropsychiatry, Psychosomatics. 2017; 9 (1S): 39–47 (in Russ.). https://doi.org/10.14412/2074-2711-2017-1S-39-47.
67. Unterberger I., Zamarian L., Prieschl M., et al. Risky decision making in juvenile myoclonic epilepsy. Front Neurol. 2018; 9: 195. https://doi.org/10.3389/fneur.2018.00195.
68. Iqbal N., Caswell H., Muir R., et al. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: an extended study. Epilepsia. 2015; 56 (8): 1301–8. https://doi.org/10.1111/epi.13061.
69. Loughman A., Bowden S.C., D'Souza W.J. A comprehensive assessment of cognitive function in the common genetic generalized epilepsy syndromes. Eur J Neurol. 2017; 24 (3): 453–60. https://doi.org/10.1111/ene.13232.
70. Giorgi F.S., Guida M., Caciagli L., et al. Social cognition in juvenile myoclonic epilepsy. Epilepsy Res. 2016; 128: 61–7. https://doi.org/10.1016/j.eplepsyres.2016.10.017.
About the Authors
K. D. LysovaRussian Federation
Kristina D. Lysova – Posrgraduate, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Professional Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: ACQ-1139-2022; Scopus Author ID: 57217092704
I. K. Kuznetsov
Russian Federation
Ivan K. Kuznetsov – Student, Faculty of Medicine, Psychology and Pharmacy
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: IZQ-3300-2023
A. I. Paramonova
Russian Federation
Anastasia I. Paramonova – Posrgraduate, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Professional Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: HMP-3496-2023
A. A. Usoltseva
Russian Federation
Anna A. Usoltseva – Posrgraduate, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Professional Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: AAM-9334-2021; Scopus Author ID: 57210425243
E. A. Kantimirova
Russian Federation
Elena A. Kantimirova – MD, PhD, Associate Professor, Chair of Medical Genetics and Clinical Neurophysiology, Institute of
Professional Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: AAJ-2986-2020
N. A. Shnayder
Russian Federation
Natalia A. Shayder – Dr. Med. Sc., Professor, Leading Researcher, Center for Collective Use “Molecular and Cellular Technologies”; Leading Researcher, Deputy Head of the Institute of Personalized Psychiatry and Neurology
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
3 Bekhterev Str., Saint Petersburg 192019, Russia
WoS ResearcherID: M-7084-2014; Scopus Author ID: 55734609800
D. V. Dmitrenko
Russian Federation
Diana V. Dmitrenko – Dr. Med. Sc., Chief of Chair of Medical Genetics and Clinical Neurophysiology, Institute of Professional Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: H-7787-2016; Scopus Author ID: 55413907300
Review
For citations:
Lysova K.D., Kuznetsov I.K., Paramonova A.I., Usoltseva A.A., Kantimirova E.A., Shnayder N.A., Dmitrenko D.V. Cognitive impairment in patients with juvenile myoclonic epilepsy. Epilepsy and paroxysmal conditions. 2024;16(1):77-87. https://doi.org/10.17749/2077-8333/epi.par.con.2024.167

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.











































