Scroll to:
Effectiveness of neuromodulation in patients with drug-resistant epilepsy after failed surgical treatment
https://doi.org/10.17749/2077-8333/epi.par.con.2024.184
Abstract
Background. In case of ineffective conservative antiepileptic therapy, surgical treatment aimed at removing the epileptogenic focus may be applied. Resection procedures allow to eliminate seizures in most patients, but in 20–30% cases they persist or recur, thereby proposing to use some neuromodulation.
Objective: to assess effectiveness of neuromodulation in patients with drug-resistant epilepsy (DRE) after failed resection surgical interventions.
Material and methods. A retrospective data analysis was carried out involving 23 DRE patients who had undergone vagus nerve stimulation (VNS) or deep brain stimulation (DBS) of the anterior nucleus of the thalamus (ANT) or hippocampus (HP) after failed surgeries. The VNS system was implanted in 18 (78.3%) patients, the HP-DBS system – in 3 (13.0%), and the ANT-DBS system – in 2 (8.7%). The results after surgical interventions were assessed according to the Engel scale, VNS therapy – by the McHugh (MH) scale, DBS therapy – by the degree of reduced seizure rate as a percentage. The average follow-up was 56.5 months.
Results. Patients with implanted VNS system were found to have the outcome presented as MH Ia–IIb in 3 (16.7%) cases, MH IIIa–IIIb in 10 (55.5%) cases, MH IV–V in 5 (27.8%) cases. In HP-DBS group, 2 out of 3 patients showed a decline in seizure rate by more than 50% from the baseline level, and 1 patient experienced an improvement in seizure severity. In the ANT-DBS group, one patient had a 60% reduction in seizure rate and an improvement in seizure severity, another one showed no change in seizure rate.
Conclusion. Neuromodulation in DRE patients can significantly lower seizure rate in more than half of patients after failed surgical treatment.
Keywords
For citations:
Efremov F.A., Agaev R.V., Kim S.A., Moisak G.I., Anisimov E.D., Khabarova E.A., Rzaev J.A. Effectiveness of neuromodulation in patients with drug-resistant epilepsy after failed surgical treatment. Epilepsy and paroxysmal conditions. 2024;16(2):96-103. https://doi.org/10.17749/2077-8333/epi.par.con.2024.184
INTRODUCTION / ВВЕДЕНИЕ
At least 50 million people worldwide suffer from epilepsy, and up to 40% of them have drug resistant epilepsy (DRE). Often, DRE shows a severe course, leading to disability and being associated with a mortality rate 5–10 times higher than in the general population. The risk of premature death in epilepsy patients exceeds that in the average population by about 3-fold [1][2].
Resection surgeries for DRE are highly effective resulting in most cases in complete seizure freedom. However, radical surgical interventions are not indicated for patients with DRE in cases of bilateral or widespread epileptic activity, when the epileptogenic zone is located near functionally significant areas, or when it is impossible to determine the localization of the seizure onset zone. Additionally, 20–30% of patients continue to have seizures after resective surgery [3] where it can be considered unsuccessful.
Currently, apart from adjustments in antiepileptic therapy and repeated surgery some form of neuromodulation can be offered for such patients, [1]. The most common types of neuromodulation for DRE today are presented as vagus nerve stimulation (VNS), responsive neurostimulation (RNS), and deep brain stimulation (DBS) of the anterior nucleus of the thalamus (ANT) or hippocampus (HP) [4].
Objective: to assess an effectiveness of neuromodulation in DRE patients after failed resection surgical interventions.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
A retrospective analysis of treatment results was conducted by assessing 23 patients (12 males and 11 females) operated for DRE at the Federal Neurosurgical Center (Novosibirsk) from 2014 to 2022, who underwent neuromodulator implantation for VNS or DBS therapy after unsuccessful surgical intervention. The data were retrieved from medical histories during follow-up visits to the center, otherwise, through telephone surveys .
Previous surgery / Первое оперативное вмешательство
All patients had previously undergone resection surgeries for PRE, and subsequently experienced seizure recurrence. Of the 23 patients, 4 (17.4%) underwent left-sided medial temporal lobectomy (MTLE), 6 (26.1%) had right-sided MTLE, 3 (13.0%) underwent selective amygdalohippocampectomy (SAHE), 6 (26.1%) had stereotactic callosotomy, 2 (8.7%) had ganglioglioma removal in the medial temporal lobe, and 2 (8.7%) had selective removal of extratemporal epileptogenic foci.
Six of the 23 patients (26.1%) underwent repeat surgery due to seizure recurrence after the first surgery. One patient underwent anterior medial temporal lobectomy after left-sided SAHE, one patient had right-sided MTLE after ganglioglioma removal in the right medial temporal lobe with right-sided SAHE, 1 patient had resection of epileptogenic foci in the right frontal lobe after right-sided MTLE, 1 patient underwent VNS implantation after right-sided MTLE, and 2 patients underwent extended temporal lobe resection after MTLE.
Neuromodulation / Нейромодуляция
The VNS system was implanted in 18 (78.3%) patients, the HP-DBS system – in 3 (13.0%), and the ANT-DBS system – in 2 (8.7%) cases. One patient had the VNS system implanted, but due to ineffective four-year-long therapy, it was removed, and replaced with the HP-DBS system.
VNS therapy
VNS implantation was performed under general anesthesia according to standard methodology, accessing the left vagus nerve along the anterior edge of the sternocleidomastoid muscle. A pocket was created in the subcutaneous tissue for the pulse generator, and the electrode was routed subcutaneously from the neck to the pocket. After placing the electrode on the vagus nerve, it was connected to the pulse generator and impedance was tested.
DBS therapy
Patients who underwent DBS received preoperative examinations, including brain magnetic resonance imaging (MRI) with T1, T2, and FGATIR sequences. DBS implantation was also performed under general anesthesia. After attaching the base frame to the patient's head, brain multislice computed tomography (MSCT) was performed with the base frame, and MSCT data were combined with MRI data obtained earlier to calculate stereotactic trajectories.
Electrodes were placed via anterior or posterior access, depending on the target (ANT or HP). After implanting and securing the electrodes, extension cables and pulse generators were implanted in the right subclavian area. HP-DBS patients received unilateral stimulation of the contralateral hippocampus. ANT-DBS stimulation was performed bilaterally via a transventricular approach.
Assessment of results / Оценка результатов
Before neuromodulator implantation, surgical outcomes were classified according to the Engel epilepsy surgery outcome scale [5].
Initial VNS programming was performed around 2 weeks after implantation, and the deep structure stimulator was programmed 1-month post-implantation. VNS therapy results were assessed using the McHugh (MH) scale, and DBS therapy results were evaluated by the percentage seizure frequency decline.
Follow-up ranged from 12 to 108 months (mean follow-up – 56.5 months) [6].
Ethical aspects / Этические аспекты
The study was conducted following the principles of the Helsinki Declaration of the World Medical Association (Fortaleza, Brazil, 2013). All patients signed an informed consent to participate in publishing the study data. Additionally, the approval of Ethical Committee at the Federal Neurosurgical Center (Novosibirsk) was obtained (Protocol No. 4 dated February 22, 2023).
Statistical analysis / Статистический анализ
Due to the small patient cohort in the study, the data are presented as descriptive statistics using percentages. All data were calculated using Office Excel (Microsoft, USA) software.
RESULTS / РЕЗУЛЬТАТЫ
Before neuromodulator implantation , the outcomes of the interventions were classified as Engel 3a in 2 patients, Engel 3b – in 2 patients, Engel 4a – in 7 patients, and Engel 4b – in 12 patients (Table 1).
Table 1. Outcomes of neuromodulation in patients with drug-resistant epilepsy
after failed resection surgery
Таблица 1. Результаты нейромодуляции у пациентов с фармакорезистентной эпилепсией
после неудачных резекционных хирургических вмешательств
Patient /Пациент | Previous surgery / Первое оперативное лечение | Baseline level by Engel scale / Исходный уровень по шкале Engel | Neuromodulation type / Вид нейромодуляции | Outcome after neurostimulator implantation** / Исход после имплантации нейростимулятора** |
1* | Right-sided MTL / Правосторонняя МВЛЭ | Engel 4b | VNS | McHugh V |
2 | Left- sided MTL, extended resection / Левосторонняя МВЛЭ, расширенная резекция | Engel 4a | ANT DBS | >60% |
3 | Right-sided MTL / Правосторонняя МВЛЭ | Engel 4a | ANT DBS | No changes / Без изменений |
4 | Right-sided MTL / Правосторонняя МВЛЭ | Engel 4a | HP DBS | >50% |
5 | Right-sided MTL, extended resection / Правосторонняя МВЛЭ, расширенная резекция | Engel 4b | HP DBS | >50% |
6 | Right-sided MTL / Правосторонняя МВЛЭ | Engel 3b | VNS | McHugh Ia |
7 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 4b | VNS | McHugh IIa |
8 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 4b | VNS | McHugh IIb |
9 | Left-sided MTL / Левосторонняя МВЛЭ | Engel 3a | VNS | McHugh IIIb |
10 | Removed focus of epileptic activity in the right parietal lobe / Удаление очага эпилептической активности теменной доли справа | Engel 4b | VNS | McHugh IIIb |
11 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 4a | VNS | McHugh IIIa |
12 | Left-sided MTL / Левосторонняя МВЛЭ | Engel 4b | VNS | McHugh IIIa |
13 | SAH, removed focus of epileptic activity in the right parietal lobe / САГЭ, удаление эпилептогенного очага теменной доли справа | Engel 4b | VNS | McHugh IIIa |
14 | Left-sided MTL / Левосторонняя МВЛЭ | Engel 4b | VNS | McHugh IIIa |
15 | Right-sided SAH, right- sided MTL / Правосторонняя САГЭ, правосторонняя МВЛЭ | Engel 4b | VNS | McHugh IIIa |
16 | Right-sided MTLE, destruction of epifoci in the right temporal lobe / Правосторонняя МВЛЭ, деструкция эпиочагов правой височной доли | Engel 3a | VNS | McHugh IIIb |
17 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 4a | VNS | McHugh IIIb |
18 | Left-sided SAH, left-sided MTL / Левосторонняя САГЭ, левосторонняя МВЛЭ | Engel 4b | VNS | McHugh IIIb |
19 | Removed ganglioglioma in the right frontoparietal area / Удаление ганглиоглиомы правой лобно-теменной области | Engel 4b | VNS | McHugh IV |
20 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 3b | VNS | McHugh V |
21 | Removed epileptogenic focus in the left parietal lobe / Удаление эпилептогенного образования левой теменной доли | Engel 4a | VNS | McHugh V |
22 | Stereotactic callosotomy / Стереотаксическая каллозотомия | Engel 4a | VNS | McHugh V |
23 | Removed ganglioglioma in the right frontal lobe / Удаление ганглиоглиомы правой лобной доли | Engel 4b | VNS | McHugh V |
Note. MTL – medial temporal lobectomy; SAH – selective amygdalohippocampectomy; VNS – vagus nerve stimulation; DBS – deep brain stimulation; ANT – anterior nucleus of the thalamus; HP – hippocampus. * Due to ineffective four-year-long VNS therapy in patient 1, the stimulator was removed and HP-DBS system was implanted (related outcome unchanged). ** VNS outcome – by McHugh scale, DBS outcome – reduced seizure rate (%).
Примечание. МВЛЭ – медиальная височная лобэктомия; САГЭ – селективная амигдалогиппокампэктомия; VNS (англ. vagus nerve stimulation) – стимуляция блуждающего нерва; DBS (англ. deep brain stimulation) – глубокая стимуляция мозга; ANT (англ. anterior nucleus of the thalamus) – переднее ядро таламуса; HP (англ. hippocampus) – гиппокамп. * Пациенту 1 в связи с неэффективностью VNS-терапии в течение 4 лет стимулятор был удален и имплантирована система HP-DBS (исход после имплантации второго нейростимулятора – без изменений). ** Исход VNS – по шкале McHugh, исход DBS – снижение частоты приступов (%).
VNS therapy / VNS-терапия
The outcome of therapy was MH Ia–IIIb in 13 cases (72.2%), of which MH Ia–IIb outcome was noted in 3 (16.7%) patients, MH IIIa–IIIb in 10 (55.5%). The MH IV–V outcome was recorded in 5 (27.8%) patients (Fig. 1).
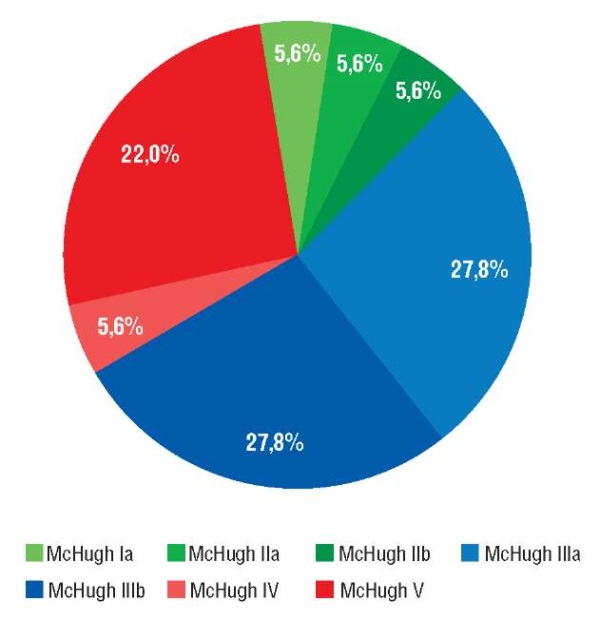
Figure 1. Outcomes after vagus nerve stimulator (VNS) implantation by McHugh scale
Рисунок 1. Исходы после имплантации системы стимуляции блуждающего нерва
(англ. vagus nerve stimulation, VNS) по шкале McHugh
DBS therapy / DBS-терапия
In the HP-DBS group, 2 patients experienced more than a 50% reduction in seizure frequency from the baseline over the course of 2-year-long observation (see Table 1). One patient did not show a decline in seizure frequency; however, severity of seizures was some alleviated, with no changes in antiepileptic therapy.
In the ANT-DBS group, one patient experienced a 60% reduction in seizure frequency and alleviated seizure severity, whereas the other patient showed no change in seizure frequency.
DISCUSSION / ОБСУЖДЕНИЕ
Currently, three neuromodulation types are most common in pharmacoresistant epilepsy (PRE): VNS, RNS, ANT-DBS, and HP-DBS.
It is assumed that the mechanism of VNS action is based on neuromodulation of the brainstem, thalamic, and cortical afferent projections, including catecholaminergic nuclei and limbic regions. The long-term effect of seizure frequency reduction via VNS therapy may also be due to other mechanisms, such as modulation of GABAergic function. The mechanism of ANT-DBS in epilepsy is still not fully understood. Chronic stimulation of the anterior nucleus of the thalamus enhances GABAergic1 neurotransmission and lowers interictal hippocampal activity in individuals with mesial temporal lobe epilepsy. The RNS system is designed for continuous monitoring of intracranial electroencephalographic patterns in the area or areas of seizure onset and for delivering short-term high-frequency stimulation upon detection of ictal patterns and preventing seizure onset [4].
Currently, there is a sufficient body of studies assessing effectiveness of neuromodulation in patients after unsuccessful surgical operations.
A.P. Amar et al. (2008) [7], based on data from the VNS Therapy Patient Outcome Registry (USA), compared groups of patients who had a VNS system implanted. In the first group (n=921), patients received surgical treatment prior to the implantation, whereas in the second group (n=3822), no surgical intervention was performed. The average reduction in seizure frequency in the first group was 42.5% after 3-month observation, increasing to 50.5% after 24 months, with 55% of therapy responders. In the second group, the average reduction in seizure frequency was 47.0% after 3-month observation and 66.7% after 24 months, with 62% of therapy responders. Analyzing the quality of life of for such patients showed a tendency for improvement over time. However, the authors note that the data obtained from the national registry may be unreliable due to systematic reporting and patient selection biases [7].
F.L. Vale et al. (2011) [4] presents the data on 37 patients with refractory epilepsy (RE) and unsatisfactory outcomes after resection surgery (lobectomy or callosotomy) who had a VNS system implanted. The reduction in seizure frequency was below 30% in 24 (64.9%) patients, 30-60% – in 9 (24.3%) patients, and more than 60% in 4 (10.8%) patients.
In 2010, the results of the SANTÉ trial (Stimulation of Anterior Nucleus of Thalamus for Epilepsy) [1] were published, conducted as a multicenter randomized controlled study with 110 patients suffering from focal refractory epilepsy (RE). All patients were implanted with a DBS system for ANT stimulation. After 3 months of the blinded phase of the study, the seizure frequency decreased by an average of 40% in the group of patients receiving stimulation, compared to a 15% decline in control group (compared to the preoperative period). However, the stimulation group experienced more frequent subjective depression and memory impairment. Interestingly, both groups experienced a reduction in seizure frequency by around 22% within the first month after implantation and before the onset of stimulation, indicating a “lesion effect” – an immediate reduction in seizure frequency caused by nerve tissue damage from electrode implantation. Subsequently, all patients underwent neurostimulation, which resulted in a progressively decreased seizure frequency by an average of 69% during 5-year observation. In 68% of patients, the seizure frequency was halved, and significantly reduced seizure severity as well as improved quality of life were observed. Subgroup analysis showed that the best results were found in temporal lobe epilepsy (during the blinded phase of the study, the average reduction in seizure frequency in this group was 44.2% compared to 40% for the entire group post-stimulation). Similarly, after 5-year follow-up, the average reduction in seizure frequency reached 76% in temporal lobe epilepsy, compared to 59% in frontal lobe epilepsy and 68% in patients with other epilepsy forms. With the recent conclusion of the study, the results of a 10-year follow-up were published: 57 of 110 patients remained active participants, with a median seizure frequency reduction of 75%, regardless of prior VNS or resection surgery. The reduction in bilateral tonic-clonic seizures was 71%. The addition of new antiepileptic drugs did not affect seizure frequency [1].
P. Ding et al. (2016) [8] present data on unilateral hippocampal stimulation in 5 patients with bitemporal DRE after unsuccessful anterior medial temporal lobectomy. In this study, 18 patients with bitemporal epilepsy underwent anterior medial temporal lobectomy (surgery was performed on the side where at least 2/3 of the seizures were registered). The number of patients without seizures post-surgery was 55.6% (10/18), 50.0% (9/18), and 44.4% (4/9) 1, 2, and 5 years later, respectively. The surgical treatment results were significantly better than in control group (12 patients) after medication therapy. Then, 5 patients underwent DBS system implantation in the hippocampus contralateral to the surgery side. During DBS therapy, seizure frequency decreased by 80–100%, and 80% of the patients were seizure-free after 1 year of observation [8].
There are several studies comparing the effectiveness of different neuromodulation methods in RE patients. J. Zhu et al. (2021) [10] describe better efficacy after ANT-DBS compared to VNS in 35 patients. Other studies (V. Shah et al. (2019) [10], T. Kulji et al. (2017) [11], J.D. Rolston et al. (2012) [12]) do not indicate a predominant effectiveness of any specific neuromodulation method. The choice of neuromodulation method is based on individual clinical characteristics such as patient age at the time of surgery, disease length, type of seizures, and the number of resective epilepsy surgeries in patient's history, as well as the patient's preferences [9–12].
In our study, both VNS and DBS therapy showed good effectiveness in reducing seizure frequency in DRE patients; however, it should be noted that the number of patients in the DBS therapy group was very small, and different targets were used. It should also be noted that some patients in the VNS therapy group underwent subsequent neurostimulation adjustments by relevant local epileptologist rather than in our center.
CONCLUSION / ЗАКЛЮЧЕНИЕ
Neuromodulation significantly lowers seizure frequency and improves quality of life in more than half of DRE patients after unsuccessful surgical treatment. According to the results of our study, the effectiveness of neurostimulation was observed in more than 50% cases. Further studies with larger patient cohorts are necessary to compare an effectiveness of various neuromodulation types.
1. GABA – gamma-aminobutyric acid.
References
1. Salanova V., Sperling M.R., Gross R.E., et al. The SANTÉ study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021; 62 (6): 1306–17. https://doi.org/10.1111/epi.16895.
2. Epilepsy. Report by the Director-General. World Health Organization. 146th session EB146/12, 25 November 2019. Available at: https://apps.who.int/gb/ebwha/pdf_files/EB146/B146_12-en.pdf (accessed 20.02.2024).
3. Ryvlin P., Rheims S., Hirsch L.J., et al. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021; 20 (12): 1038–47. https://doi.org/10.1016/S1474-4422(21)00300-8.
4. Vale F.L., Ahmadian A., Youssef A.S., et al. Long-term outcome of vagus nerve stimulation therapy after failed epilepsy surgery. Seizure. 2011; 20 (3): 244–8. https://doi.org/10.1016/j.seizure.2010.12.003.
5. Engel J.V.N.P. Jr., Rasmussen T.B., Ojemann L.M. (Eds.) Outcome with respect to epileptic seizures. New York: Raven Press; 1993: 609–21.
6. McHugh J.C., Singh H.W., Phillips J., et al. Outcome measurement after vagal nerve stimulation therapy: proposal of a new classification. Epilepsia. 2007; 48 (2): 375–8. https://doi.org/10.1111/j.1528-1167.2006.00931.x.
7. Amar A.P., Apuzzo M.L., Liu C.Y. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004; 55 (5): 1086–93. https://doi.org/10.1227/01.neu.0000141073.08427.76.
8. Ding P., Zhang S., Zhang J., et al. Contralateral hippocampal stimulation for failed unilateral anterior temporal lobectomy in patients with bilateral temporal lobe epilepsy. Stereotact Funct Neurosurg. 2016; 94 (5): 327–35. https://doi.org/10.1159/000449008.
9. Zhu J., Wang X., Xu C., et al. Comparison of efficiency between VNS and ANT-DBS therapy in drug-resistant epilepsy: a one year follow up study. J Clin Neurosci. 2021; 90: 112–7. https://doi.org/10.1016/j.jocn.2021.05.046.
10. Shah V., Eliashiv D., Reider-Demer M. Neurostimulation & Epilepsy. For medically refractory epilepsy, neurostimulation options – old and new – provide new hope. Available at: https://practicalneurology.com/articles/2019-oct/neurostimulation-epilepsy/pdf (accessed 20.02.2024).
11. Kulju T., Haapasalo J., Lehtimäki K., et al. Similarities between the responses to ANT-DBS and prior VNS in refractory epilepsy. Brain BehaV. 2018; 8 (6): e00983. https://doi.org/10.1002/brb3.983.
12. Rolston J.D., Englot D.J., Wang D.D., et al. Comparison of seizure control outcomes and the safety of vagus nerve, thalamic deep brain, and responsive neurostimulation: evidence from randomized controlled trials. Neurosurg Focus. 2012; 32 (3): E14. https://doi.org/10.3171/2012.1.FOCUS11335.
About the Authors
F. A. EfremovRussian Federation
Fedor A. Efremov – Neurosurgeon, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
R. V. Agaev
Russian Federation
Rasim V. Agaev – Neurosurgeon, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
S. A. Kim
Russian Federation
Sergei А. Kim – Neurosurgeon, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
G. I. Moisak
Russian Federation
Galina I. Moisak – MD, PhD, Neurologist, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
Scopus Author ID 57088972500
E. D. Anisimov
Russian Federation
Egor D. Anisimov – Neurosurgeon, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
E. A. Khabarova
Russian Federation
Elena A. Khabarova – Neurologist, Department of Functional Neurosurgery, Federal Neurosurgical Center.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087
J. A. Rzaev
Russian Federation
Jamil A. Rzaev – MD, PhD, Chief Physician, Federal Neurosurgical Center; Associate Professor, Chair of Neuroscience, Institute of Medicine and Psychology, Novosibirsk State Medical University.
132/1 Nemirovich-Danchenko Str., Novosibirsk 630087; 52 Krasnyy Ave, Novosibirsk 630091
Review
For citations:
Efremov F.A., Agaev R.V., Kim S.A., Moisak G.I., Anisimov E.D., Khabarova E.A., Rzaev J.A. Effectiveness of neuromodulation in patients with drug-resistant epilepsy after failed surgical treatment. Epilepsy and paroxysmal conditions. 2024;16(2):96-103. https://doi.org/10.17749/2077-8333/epi.par.con.2024.184
JATS XML

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.







































