Scroll to:
Сlinical and neuroimmunological correlations in post-stroke epilepsy illustrated by analyzing serum neuron-specific enolase and vascular endothelial growth factor
https://doi.org/10.17749/2077-8333/epi.par.con.2024.205
Abstract
Background. Due to progress in the treatment of patients who have suffered a stroke, the prevalence of post-stroke epilepsy (PSE) has been increasing. The search for biomarkers that determine the prognosis of ischemic stroke (IS) complications and PSE development along with creating a diagnostic protocol subsequently is useful for advancing tactics of PSE therapy.
Objective: to investigate the blood serum levels of neuron-specific enolase (NSE) and vascular endothelial growth factor (VEGF) in PSE patients paralleled by assessing clinical and neuroimmunological correlations.
Material and methods. A total of 140 patients aged 28 to 84 years with the first IS was examined. Of these, 70 patients newly developed late epileptic seizures (main group), 70 patients had IS without epileptic seizures (comparison group). The control group consisted of 30 patients without IS or epilepsy. IS severity was assessed according to the National Institutes of Health Stroke Scale (NIHSS), the degree of disability – according to the modified Rankin Scale (mRS), the level of patient’s basic functional activity – according to the Barthel Index (BI). Prediction of post-IS onset of late seizures was performed according to the SeLECT scale (SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery). To assess severity of epilepsy, the K. Lühdorf et al. classification was used. The levels of NSE neurotrophic factor and VEGF angiogenesis factor were measured in blood serum samples from all patients by using enzyme-linked immunosorbent assay (ELISA).
Results. A significantly increased NSE and VEGF levels were noted in main group (by 4.72- and 1.59-fold, respectively) and in comparison group (by 4.45- and 1.54-fold, respectively) compared to control group. In addition, NSE and VEGF levels in main group significantly exceeded those in comparison group (by 1.06- and 1.03-fold, respectively). Both biomarkers also tended to increase in patients with moderate and severe PSE. The level of NSE/VEGF correlation characterizing damage to the nervous tissue and angiogenesis as well as degree of severity, disability, rehabilitation potential, patients’ everyday life activity, NSE and VEGF prognostic significance in development and severity level of epilepsy in IS patients with epileptic seizures was determined.
Conclusion. NSE and VEGF hyperexpression is important in predicting development or progression (worsening) of epilepsy after IS.
Keywords
For citations:
Rakhimbaeva G.S., Sobirova D.S. Сlinical and neuroimmunological correlations in post-stroke epilepsy illustrated by analyzing serum neuron-specific enolase and vascular endothelial growth factor. Epilepsy and paroxysmal conditions. 2024;16(4):316-326. https://doi.org/10.17749/2077-8333/epi.par.con.2024.205
INTRODUCTION / ВВЕДЕНИЕ
Cerebrovascular diseases are a leading cause of epilepsy in older adults. Acute cerebrovascular accidents (ACVAs) account for approximately 10% of all epilepsy cases and 55% of newly diagnosed seizures in the elderly. The majority of strokes occur in individuals over the age of 45 years. Up to 90% of these strokes are ischemic (IS), while the remaining 10% are hemorrhagic [1][2].
Post-stroke epilepsy (PSE), a form of acquired structural epilepsy, can lead to complications that diminish quality of life, increase mortality, and elevate healthcare costs [3]. Approximately 10% of individuals who have experienced a stroke develop PSE. Given the substantial global stroke burden, PSE represents a significant issue for stroke survivors [2]. Furthermore, 40% of late-onset epilepsy cases are associated with cerebrovascular diseases, whereas late-onset epilepsy is often accompanied by recurrent ACVAs, underscoring its importance both globally and nationally.
The risk of developing epilepsy increases in individuals experiencing long-term IS sequelae [4][5]. According to M.Y. Xu (2018), early epileptic seizures occur within the first 2 days following stroke, while late seizures may manifest between the 6-month and 1-year post-stroke [6]. Initial seizures, typically occurring within the first 7 days of the acute stroke period, late seizures (from day 8 to day 21 of the acute stroke stage), and prodromal seizures (preceding stroke) are classified according to G.S. Barolin’s classification (1962) [7]. According to A. Pezzini et al. (2024) [1] as well as H. Stefan and G. Michelson (2024) [4], the frequency of early epileptic seizures in post-stroke patients ranges from 2–33% to 50–78%, while late post-stroke seizures are found in 3–4.5% to 67% of cases. However, their role in developing PSE remains understudied. The mechanisms of PSE formation, key predictors of post-stroke epileptic seizures, and the role for various biomarkers in underlying pathogenesis along with an impact on disease outcomes remain unclear.
Various measurable biomarkers may indicate the development of post-ACVA epilepsy. During the biological process of neuroinflammation, inflammatory mediators such as prostaglandins, chemokines, cytokines, complement components, growth factors, and damage-associated molecular patterns (DAMPs) are released [8–10]. Prolonged neuroinflammation leads to increased excitability, changes in synaptic transmission, gliosis, disruption of the blood-brain barrier (BBB), and abnormal neurogenesis [11–14]. Alterations in blood biomarker concentrations may indicate the presence or progression of disease [15][16], and some of such biomarkers exhibit neuroprotective properties [16][17]. Therefore, investigating such issues is of considerable relevance.
The study of neuron-specific enolase (NSE) has emerged as a promising avenue in neuroimmunology for diagnosing various neurodegenerative disorders of the central nervous system (CNS), including epilepsy. In the study by G.S. Rakhimbayeva and N.S. Rashidova (2011), the serum NSE levels in patients with post-traumatic epilepsy were found to be higher than those in patients with vascular epilepsy [18]. Moreover, NSE level peaked in patients with more frequent seizures, which is particularly evident in younger individuals, as reported by X. Bai et al. (2022) [19] and H. Eriksson et al. (2021) [20].
Vascular endothelial growth factor (VEGF) is another molecule linked to the blood-brain barrier (BBB) dysfunction [16][21–23]. Angiogenesis, a de novo blood vessel formation, can occur under both physiological (e.g., during development) and pathological conditions [24]. The local VEGF overexpression underlies the formation and progression of vascular malformations [25–27]. V. Rigau et al. (2007) demonstrated that hippocampal vascular density in patients with epilepsy is approximately twice as high as in non-epileptic control groups [22]. However, how VEGF-induced BBB dysfunction contributes to developing epilepsy and epileptic seizures remains unclear.
Thus, due to the advanced treatment strategies for patients who have experienced a stroke, the PSE prevalence has been increasing. Maintaining vigilance regarding the onset of PSE, as well as its prevention and early diagnostics, is of considerable practical importance. The identification of biomarkers that predict IS complications and PSE emergence accompanied by developing a diagnostic protocol, holds both scientific and practical interest.
Objective: to investigate the blood serum NSE and VEGF levels in PSE patients paralleled by assessing clinical and neuroimmunological correlations.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
Between 2020 and 2024, a total of 170 patients aged 28 to 84 years took part in the study conducted at the multidisciplinary clinic of the Tashkent Medical Academy and the City Clinical Hospital No. 7 in Tashkent (Uzbekistan).
Inclusion and exclusion criteria / Критерии включения и исключения
When compiling the study cohort, the following inclusion criteria were taken into account:
– age over 18 years;
– late recovery and residual phases following IS (for IS groups).
Exclusion criteria:
– сhildhood and adolescence;
– hyperacute and acute periods of IS;
– hemorrhagic stroke;
– hypertensive crises and transient ischemic attacks;
– epilepsy preceding the stroke;
– tumors, traumatic brain injuries;
– toxic lesions;
– clinical death;
– brain inflammatory diseases (meningitis, meningoencephalitis, encephalitis, leptomeningitis, leukodystrophies, neurosyphilis, Guillain–Barré syndrome);
– brain demyelinating diseases (multiple sclerosis, acute disseminated encephalomyelitis, myasthenia, polyneuropathies);
– mental disorders.
Patient groups / Группы пациентов
Patients who had experienced IS were divided into two groups based on the presence or absence of epilepsy.
Main group (Group 1) included 70 patients (42 (60%) males and 28 (40%) females) who had late epileptic seizures following first IS, with mean age 62.95±9.98 years (ranging from 28 to 79 years, median 63 years).
Comparison group (Group 2) consisted of 70 patients (51 (72.86%) males and 19 (27.14%) females) with IS sequelae but no epileptic seizures, with mean age 62.24±11.55 years (ranging from 30 to 84 years, median 62 years).
Control group included 30 patients (9 (42.9%) males and 12 (57.1%) females) with astheno-neurotic syndrome and dorsopathy, without history of IS, epilepsy, or other severe somatic or neurological diseases, with mean age 55.18±10.48 years (ranging from 45 to 59 years, median 55 years).
Diagnostics / Диагностика
All patients underwent neuroimaging (brain magnetic resonance imaging (MRI) and computed tomography (CT)), neurophysiological examination (electroencephalography (EEG)), as well as clinical and neurological evaluations at the time of primary examination, followed by 6–12 month-observation.
MRI scans were performed using a Phillips American G machine (1.5 T magnetic field strength) with T1, T2, FLAIR, FSE-axial, FSE-coronal, T2*/SWI, and DWI sequences. Non-contrast CT and CT perfusion studies were conducted with a Revolution 128-slice multislice CT scanner (Philips, Netherlands) using an anterior-posterior scanning method with a slice thickness of 2.5 mm, and carried out at the private clinic “Medion” in Tashkent. All patients underwent EEG recording using a 19-channel Neuron-Spectrum-3 system (Neurosoft, Russia).
The diagnosis of IS and its sequelae was made in accordance with the International Classification of Diseases, Tenth Revision (ICD-10) (code I69.3 for IS sequelae), as well as widely accepted the European Stroke Organization (ESO) [28] and the International League Against Epilepsy (ILAE) [29, 30] guidelines.
The classification of epileptic seizures and epilepsy was based on the ILAE’s 2017 operational classification of seizure types and the definition of epilepsy [29, 30]. In addition, ICD-10 was used to confirm the diagnosis of epilepsy (code: G40.
Методы оценки / Assessment methods
The severity of IS was assessed by using the National Institutes of Health Stroke Scale (NIHSS), the degree of disability – by the modified Rankin Scale (mRS), and the effectiveness of care and basic patient functional activity – by the Barthel Index (BI).
To predict late seizures following IS, the SeLECT score (SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery) was employed [31], which can aid in preventing post-stroke late epileptic seizures. It takes into account early seizures, stroke severity (by NIHSS score of 11 or higher), as well as cortical and arterial territory involvement, including the cerebral cortex, middle cerebral artery territory, and large-artery atherosclerotic lesions to predict likelihood for seizure onset in post-stroke patients within 5 years. An early seizure, which is not classified as epilepsy, does not indicate PSE. Although there is an association between early seizures and an increased risk of developing epilepsy in the future, this does not justify a PSE diagnosis [31]. The studies by E. Beghi et al. (2011) [32] and M. Holtkamp et al. (2017) [33] demonstrated that a seizure occurring more than one week post-stroke is classified as late and unprovoked, markedly increasing the probability of recurrence. Such a seizure raises the risk of recurrent seizures by more than 60%.
To evaluate the severity of epilepsy, the classification proposed by K. Lühdorf et al. (1986) was used: mild severity – 3 or fewer epileptic seizures per year; moderate severity – 12 or fewer seizures per year; and severe severity – 13 or more seizures per year [34].
Enzyme-linked immunosorbent assay / Иммуноферментный анализ
In addition to standard tests, serum NSE and VEGF levels were measured by enzyme-linked immunosorbent assay (ELISA) in all patients from main and comparison groups who had experienced IS more than 6 months before, as well as in control group. NSE levels were assessed by using the “NSE-IFA” reagent kit (Hema LLC, Russia), and VEGF levels – by using the “VEGF-IFA-BEST” reagent kit (Vector-Best JSC, Russia), in accordance with the manufacturers’ instructions.
Statistical analysis / Статистический анализ
Baseline data were recorded on patient registration cards to be transferred into an electronic database using Excel 2010 (Microsoft, USA). Traditional methods of descriptive and inferential statistics were employed. Results are presented as mean ± standard deviation (M±SD). After verifying the normality of distribution, quantitative variables were assessed using the Student’s t-test, whereas qualitative variables were assessed by applying the χ2 test. Pearson’s correlation coefficient (r) was calculated to analyze a relationship between two sample sets. The differences were considered statistically significant at significance level of at least 95% (p<0.05).
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Assessment by scales / Оценка по шкалам
The results of applying the assessment scales in both IS patient groups are presented in Table 1.
Stroke severity
In main group, the NIHSS score was 20.1±4.0 points, which was 1.04-fold higher than in comparison group, where the total score was 19.37±4.46 (p=0.3) (Table 2). In Group 1, 22.86% of patients were diagnosed with mild IS (mean NIHSS score 9.69±2.9), 35.71% with moderate-to-severe IS (mean score 18.36±1.65), and 41.43% with severe IS (mean score 27.46±5.93). In Group 2, 27.14% of patients had mild IS (mean score 12.00±2.93), 45.72% had moderate-to-severe IS (mean score 19.0±1.3), and 27.14% had severe IS (mean score 24.68±4.07). Thus, severe IS was more frequently observed in patients with PSE (p<0.05).
Table 1. Results of patient assessments according to various scales in the study groups, score
Таблица 1. Результаты оценки состояния пациентов по различным шкалам в группах исследования, баллы
Scale / Шкала | Main group / Основная группа (n=70) | Comparison group / Группа сравнения (n=70) | р |
NIHSS | 20,10±4,00 | 19,37±4,46 | 0,300 |
mRS | 3,00±1,00 | 2,27±0,96 | <0,001 |
BI | 65,00±12,65 | 65,60±9,32 | 0,700 |
SeLECT | 3,41±1,30 | 2,94±1,30 | 0,030 |
Note. NIHSS – National Institutes of Health Stroke Scale; mRS – modified Rankin Scale; BI – Barthel Index; SeLECT (SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery) – a scale for predicting late epilepsy associated with ischemic stroke.
Примечание. NIHSS (англ. National Institutes of Health Stroke Scale) – шкале оценки тяжести инсульта Национальных институтов здравоохранения США; mRS (англ. modified Rankin Scale) – модифицированная шкала Рэнкина;
BI (англ. Barthel Index) – индекс Бартела; SeLECT (англ. SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery) – шкала для прогнозирования поздней эпилепсии, связанной с ишемическим инсультом (учитывает тяжесть инсульта, атеросклероз крупных артерий, ранние приступы, поражение коры головного мозга, область средней мозговой артерии).
Table 2. Distribution of patients according to stroke severity level based on the National Institutes of Health Stroke Scale (NIHSS)
Таблица 2. Распределение больных в зависимости от степени тяжести инсульта по шкале инсульта Национальных институтов здравоохранения США (англ. National Institutes of Health Stroke Scale, NIHSS)
Stroke severity / Степень тяжести инсульта | Main group / Основная группа (n=70) | Comparison group / Группа сравнения (n=70) | p | ||
n (%) | Mean score / Средний балл | n (%) | Mean score / Средний балл | ||
Mild / Легкий | 16 (22,86) | 9,69±2,90 | 19 (27,14) | 12,00±2,93 | 0,001 |
Moderate / Среднетяжелый | 25 (35,71) | 18,36±1,65 | 32 (45,72) | 19,00±1,30 | >0,050 |
Severe / Тяжелый | 29 (41,43) | 27,46±5,93 | 19 (27,14) | 24,68±4,07 | <0,050 |
Total / Итого | 70 (100,00) | 20,10±4,00 | 70 (100,00) | 19,37±4,46 | 0,300 |
Degree of disability
Using the mRS, patients with IS and PSE demonstrated higher disability levels compared to those without PSE (3.00±1.00 vs. 2.27±0.96 points, respectively; p<0.001) (see Table 1). Рatients with vs. without PSE have a significantly higher mRS score. Patients without PSE require no external assistance (<3 points on the mRS), whereas patients with PSE do require it (≥3 points on the mRS). Thus, it may be assumed that epilepsy may worsen the condition of patients with stroke.
Daily life activity
The BI scores for daily living activities averaged 65.0±12.65 points in Group 1 and 65.6±9.32 points in Group 2 (see Table 1), suggesting about a moderate level of external dependency for daily activities. No significant inter-group differences assessing this criterion were found (p=0.7).
Risk of developing late seizures
Before treatment, the mean SeLECT score in Group 1 and Group 2 was 3.41±1.30 and 2.94±1.30 (p=0.03), respectively (see Table 1). The two groups differed significantly by a factor of 1.16, which may suggest a higher risk of developing late seizures in IS patients who meet the above-mentioned criteria.
Severity of epilepsy
Among the patients, 32 (45.71%) had mild severity PSE, 12 (17.14%) – moderate severity (p<0.001), and 26 (37.15%) – severe severity (p<0.05) (Fig. 1). Taking into account that the classification by K. Lühdorf et al. is solely based on the frequency but not on the form of epileptic seizures (simple partial, complex partial, generalized or secondary generalized), a more severe course of PSE was certainly observed in patients with a high frequency, primary generalized forms of seizures and epileptic status. Thus, in this PSE patient cohort, mild and severe PSE were most often recorded, depending on the frequency and form of epileptic seizures.
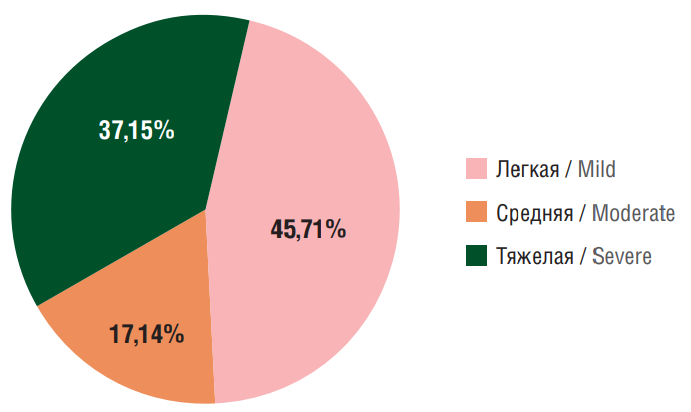
Figure 1. Distribution of patients with post-stroke epilepsy stratified by epilepsy severity level
Рисунок 1. Распределение больных с постинсультной эпилепсией по степени тяжести течения эпилепсии
Enzyme-linked immunosorbent assay / Иммуноферментный анализ
NSE levels
Next, NSE serum levels were analyzed. During the studied IS period, an increase in NSE concentration was observed. The average NSE levels in main and comparison groups were 18.42±3.0 ng/mL and 17.34±3.37 ng/ml, respectively compared to 3.9±1.3 ng/ml in control group, representing 4.72- and 4.45-fold higher levels, respectively (p<0.001) (Table 3).
At the same time, the NSE level in Group 1 exceeded that in Group 2 by a factor of 1.06 (p=0.04) (Fig. 2). The highest NSE values were observed in patients with moderate-to-severe and severe IS in both groups (p<0.05) suggesting that it may serve as a predictor of neurological deficits and development of epilepsy following IS.
Conducting a correlation analysis between NSE levels and assessment scale scores in patients with PSE allowed to identify a strong positive correlation between NSE levels and NIHSS scores (r=0.71) (Fig. 3a) suggesting that an increase in NSE levels serves as a marker of severe IS and unfavorable prognosis in patients with PSE.
In patients with PSE, a moderate positive correlation was observed between NSE concentration and mRS scores (r=0.50) (Fig. 3b), suggesting that elevated NSE levels may negatively affect the rehabilitation period.
A weak correlation was found between NSE levels and BI scores (r=0.31) (Fig. 3c). The BI value mirrors a patient’s activity and external dependency in daily life activities. Thus, high NSE levels may indirectly affect activity of PSE patients by exacerbating neurological deficits. These results in line with those reported elsewhere [18][35].
The correlation coefficient between NSE levels and SeLECT scores was r=0.88, indicating a strong positive relationship (Fig. 3d). Considering that the lowest SeLECT score predicts a risk of late seizures within 1 year post-stroke at 0.7% and within 5 years at 1.3%, while the highest score (9 points) predicts a risk of late seizures at 63% within 1 year and 83% within 5 years [31], as well as the direct relationship between higher SeLECT scores and elevated NSE levels, it can be asserted that both the SeLECT score and NSE are valid predictors of late epileptic seizure risk after IS.
NSE is a CNS intracellular enzyme and enolase isoform, which is essential for glycolysis. This marker is used to assess CNS differentiation and is one of the most specific indicators of neuronal damage. Beginning at synaptogenesis, around at gestational age of week 22, NSE becomes apparent in later stages of neuronal differentiation. This unique serum marker can also be used to detect neural tissue tumors and neuroendocrine neoplasms [35]. Increased serum NSE levels are observed in ischemic injury, brain trauma, epilepsy, and subarachnoid hemorrhage [36][37].
Thus, patients with stroke sequelae and epileptic seizures show a 1.06-fold higher serum NSE concentration compared to IS patients without epilepsy and a 4.72-fold higher level compared to control group indicating a more profound neuronal damage in patients with PSE. Consequently, the use of NSE as a marker of brain damage may strengthen the diagnosis and prognosis of PSE outcomes.
Table 3. Levels of patient serum neuron-specific enolase (NSE) and vascular endothelial growth factor (VEGF)
Таблица 3. Уровни нейронспецифической енолазы (англ. neuron-specific enolase, NSE) и фактора роста эндотелия сосудов (англ. vascular endothelial growth factor, VEGF) в сыворотке крови пациентов
Biomarker / Биомаркер | Main group / (n=70) | Comparison group /Группа сравнения (n=70) | Control / Контроль (n=30) | р |
NSE, ng/ml // NSE, нг/мл | 18,42±3,00 | 17,34±3,37 | 3,9±1,3 | <0,001 |
VEGF, pg/ml // VEGF, пг/мл | 286,75±17,40 | 278,20±12,08 | 180,1±12,1 | <0,001 |
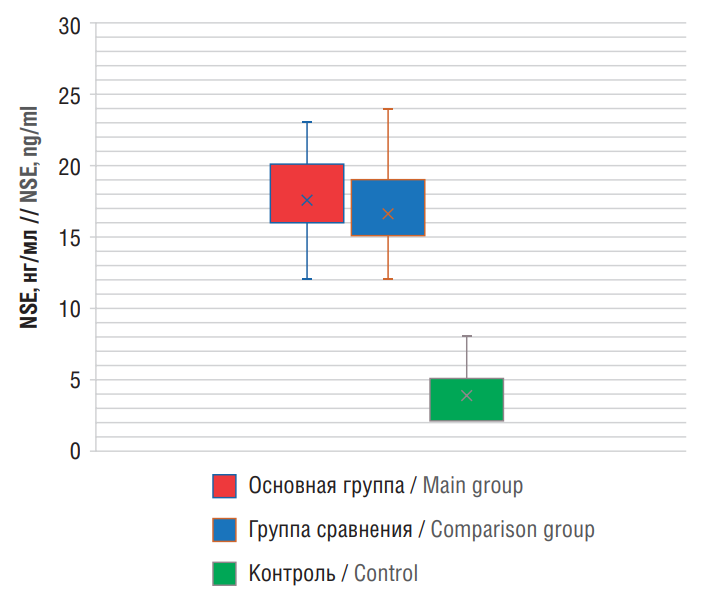
Figure 2. Levels of patient serum neuron-specific enolase (NSE)
Рисунок 2. Уровни нейронспецифической енолазы (англ. neuron-specific enolase, NSE) в сыворотке крови пациентов
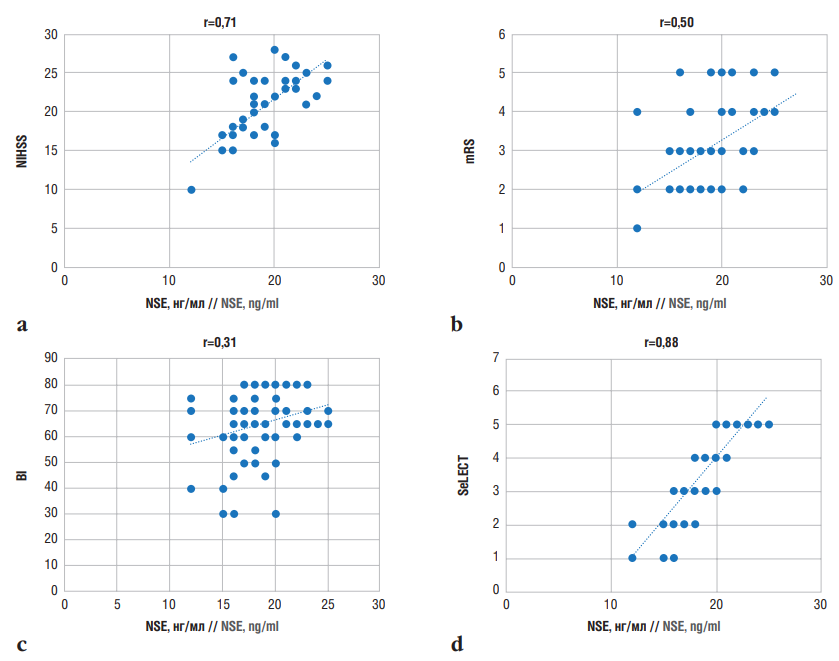
Figure 3. Correlations between neuron-specific enolase (NSE) levels in post-stroke epilepsy and assessment scale scores:
a – National Institutes of Health Stroke Scale (NIHSS); b – modified Rankin Scale (mRS); c – Barthel Index (BI); d – SeLECT (SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery) score
Рисунок 3. Корреляционные взаимосвязи между уровнем нейронспецифической енолазы (англ. neuron-specific enolase, NSE) при постинсультной эпилепсии и баллами по оценочным шкалам:
а – шкала оценки тяжести инсульта Национальных институтов здравоохранения США (англ. National Institutes of Health Stroke Scale, NIHSS); b – модифицированная шкала Рэнкина (англ. modified Rankin Scale, mRS); с – индекс Бартела
(англ. Barthel Index, BI); d – шкала SeLECT (англ. SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery)
VEGF levels
ELISA-based analysis revealed that serum VEGF concentrations in main and comparison groups were significantly increased by 1.59-fold (286.75±17.40 pg/ml) and 1.54-fold (278.20±12.08 pg/ml), respectively compared to control group (180.1±2.1 pg/ml) (p<0.001) (see Table 3).
Moreover, VEGF levels in Group 1 were significantly higher than those in Group 2 by a factor of 1.03 (p<0.001) (Fig. 4). It was also demonstrated that VEGF hyperexpression is more common in patients with epilepsy and encephalopathy suggesting that VEGF is involved not only in angiogenesis (de no blood vessel formation) but also in epileptogenesis. “Vascular epilepsy” is associated with VEGF hyperexpression. Other studies have also highlighted VEGF role in epilepsy pathogenesis [21][22].
Correlation analysis revealed a weak positive relationship (r=0.47) between VEGF levels and NIHSS scores (Fig. 5a), indicating a limited association between VEGF hyperexpression and IS severity. The dual role of VEGF in the pathogenesis of IS has also been reported in studies suggesting that VEGF hyperexpression plays a compensatory role during the acute phase of the disease by promoting outgrowth of new blood vessels [16][24].
A weak positive correlation was identified between mRS scores and VEGF levels in patients with PSE (r=0.44) (Fig. 5b) implying that VEGF levels are weakly associated with the degree of disability and rehabilitation potential in PSE patients.
Similarly, weak correlations were observed between VEGF levels and BI scores (r=0.33) (Fig. 5c) and SeLECT scores (r=0.40) (Fig. 5d). These findings indicate that elevated VEGF levels are not strongly associated with the development of late epilepsy after IS or with patients’ daily living activities. Moreover, VEGF levels have no significant impact on predicting the development or worsening of epilepsy during the studied post-stroke period.
To further investigate a relationship between VEGF and NSE levels in PSE, a correlation analysis was performed, revealing a weak positive correlation (r=0.38) (Fig. 6).
Formation and progression of vascular anomalies such as arteriovenous malformations (AVMs) [25] is coupled to VEGF hyperexpression detected in astrocytes, neurons, and endothelial cells. Consequently, these cell types may secrete VEGF to support angiogenesis and AVM formation [21][25], although direct evidence corroborating this hypothesis is lacking.
Angiogenesis in the brain of epilepsy patients correlates with BBB dysfunction associated with VEGF [21]. VEGF and its receptor 2 (vascular endothelial growth factor receptor 2, VEGFR2) mRNA expression levels were upregulated, whereas that of ZO-1, a tight junction-associated protein, was downregulated in kainic acid-treated slice cultures [26]. Treatment with anti-VEGF antibodies mitigated the increase in vessel density, the number of vascular branches, and the reduction in ZO-1 expression. It has also been found that VEGF/VEGFR2 signaling likely induces angiogenesis and BBB dysfunction via the proto-oncogene tyrosine kinase Src pathway [21][26].
Endothelial cells express VEGFR, and several authors suggest that matrix metalloproteinase activates the VEGF/VEGFR signaling pathway in angiogenesis under pathological conditions [21][26][27]. However, whether and how these cascades contribute to angiogenesis in the brain of epilepsy patients remains to be further explored.
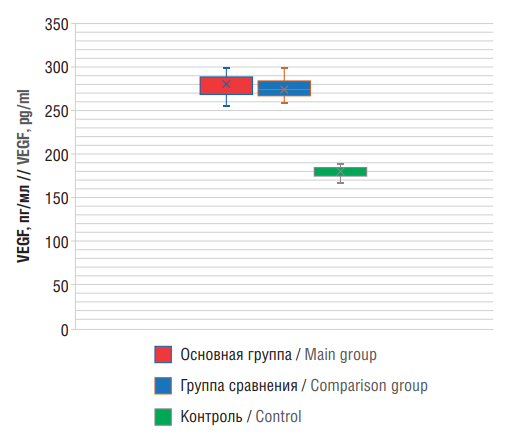
Figure 4. Levels of patient serum vascular endothelial growth factor (VEGF)
Рисунок 4. Уровни фактора роста эндотелия сосудов (англ. vascular endothelial growth factor, VEGF) в сыворотке крови пациентов
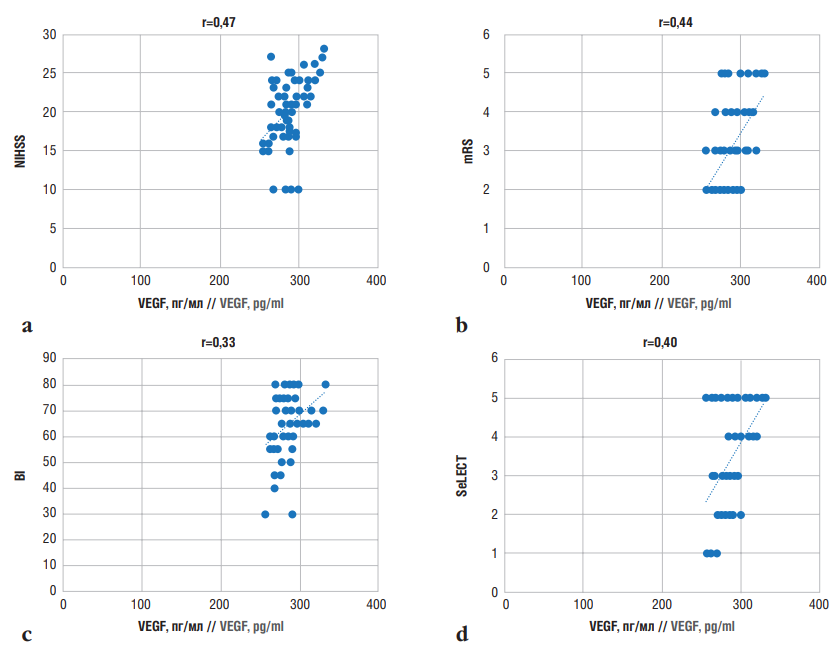
Figure 5. Correlations between vascular endothelial growth factor (VEGF) levels in post-stroke epilepsy and assessment scale scores: a – National Institutes of Health Stroke Scale (NIHSS); b – modified Rankin Scale (mRS); c –Barthel Index (BI); d – SeLECT (SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery) score
Рисунок 5. Корреляционные взаимосвязи между уровнем фактора роста эндотелия сосудов (англ. vascular endothelial growth factor, VEGF) при постинсультной эпилепсии и баллами по оценочным шкалам: а – шкала оценки тяжести инсульта Национальных институтов здравоохранения США (англ. National Institutes of Health Stroke Scale, NIHSS); b – модифицированная шкала Рэнкина (англ. modified Rankin Scale, mRS); с – индекс Бартела
(англ. Barthel Index, BI); d – шкала SeLECT (англ. SEverity of stroke, Large artery atherosclerosis, Early seizure, Cortical involvement, Territory of the middle cerebral artery)
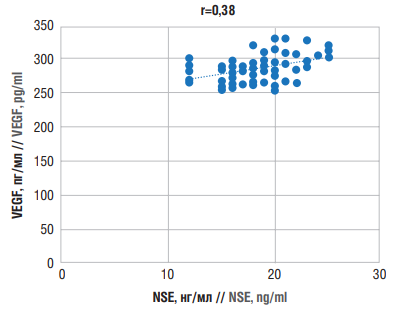
Figure 6. Correlations between neuron-specific enolase (NSE) and vascular endothelial growth factor (VEGF) levels in post-stroke epilepsy
Рисунок 6. Корреляционные взаимосвязи между уровнями нейронспецифической енолазы (англ. neuron-specific enolase, NSE) и фактора роста эндотелия сосудов (англ. vascular endothelial growth factor, VEGF) при постинсультной эпилепсии
CONCLUSION / ЗАКЛЮЧЕНИЕ
Here, we demonstrated that NSE hyperexpression, a marker of brain damage, is crucial in diagnosing and predicting PSE outcomes. VEGF hyperexpression plays a dual role in PSE pathogenesis attributed to the IS phase and the presence of epilepsy. VEGF exhibits a compensatory function during the acute phase of IS but serves as an unfavorable indicator during the chronic phase, particularly in case of vascular and structural epilepsy, suggesting its involvement in epileptogenesis.
The correlation between markers indicative of neuronal damage and angiogenesis and the severity of disease, disability, rehabilitation potential, and daily life activity of patients was established. Additionally, the prognostic significance for NSE and VEGF hyperexpression in development and progression of epilepsy in patients with seizures and stroke sequelae was confirmed, which may be used to predict development or worsening of post-stroke epilepsy.
References
1. Pezzini A., Tarantino B., Zedde M., еt al. Early seizures and risk of epilepsy and death after intracerebral haemorrhage: The MUCH Italy. Eur Stroke J. 2024; 9 (3): 630–8. https://doi.org/10.1177/23969873241247745.
2. Tanaka T., Ihara M., Fukuma K., et al. Pathophysiology, diagnosis, prognosis, and prevention of poststroke epilepsy: clinical and research implications. Neurology. 2024; 102 (11): e209450. https://doi.org/10.1212/WNL.0000000000209450.
3. Liang M., Zhang L., Geng Z. Advances in the development of biomarkers for poststroke epilepsy. Biomed Res Int. 2021; 2021: 5567046. https://doi.org/10.1155/2021/5567046.
4. Stefan H., Michelson G. Late onset epilepsy and stroke: diagnosis, pathogenesis and prevention. Seizure. 2024: S1059–1311(24)00168-7. https://doi.org/10.1016/j.seizure.2024.06.011.
5. Grigolashvili M.A., Zhuanysheva E.M. Risk factors for post stroke epilepsy. S.S. Korsakov Journal of Neurology and Psychiatry. 2021; 121 (8-2): 35-–40 (in Russ.). https://doi.org/10.17116/jnevro202112108235.
6. Xu M.Y. Poststroke seizure: optimising its management. Stroke Vasc Neurol. 2018; 4 (1): 48–56. https://doi.org/10.1136/SVN-2018-000175.
7. Barolin G.S., Sherzer E. Epileptic seizures in apoplectic patients. Wein Nervenh. 1962; 20: 35–47 (in German).
8. Madjidova Y.N., Rakhimbaeva G.S., Azizova R.B. Neuroimmunopathogenic mechanisms of epilepsy. Epilepsia i paroksizmal'nye sostoania / Epilepsy and Paroxysmal Conditions. 2014; 6 (1): 15–8 (in Russ.).
9. Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019; 15 (8): 459–72. https://doi.org/10.1038/s41582-019-0217-x.
10. Wang M., Yu J., Xiao X., et al. Changes of biochemical biomarkers in the serum of children with convulsion status epilepticus: a prospective study. BMC Neurol. 2022; 22 (1): 196. https://doi.org/10.1186/s12883-022-02686-2.
11. Abraira L., Santamarina E., Cazorla S., еt al. Blood biomarkers predictive of epilepsy after an acute stroke event. Epilepsia. 2020; 61 (10): 2244–53. https://doi.org/10.1111/epi.16648.
12. Abraira L., López-Maza S., Quintana M., еt al. Exploratory study of blood biomarkers in patients with post-stroke epilepsy. Eur Stroke J. 2024; 9 (3): 763–71. https://doi.org/10.1177/23969873241244584.
13. Rasulova Kh.A., Rasulova M.A. Immune analysis of natural neurotropic autoantibodies in blood serum of patients with COVID-19 associated ischemic stroke. Medical Immunology (Russia). 2024; 26 (6): 1257–68 (in Russ.). https://doi.org/10.15789/1563-0625-IAO-2888.
14. Rasulova K.A., Arizova R.B. Natural neurotropic autoantibodies in blood serum of epilepsy patients. Vestnik Rossiiskoi akademii medetsinskikh nauk / Annals of the Russian Academy of Medical Sciences. 2014; 69 (5–6): 111–6 (in Russ.). https://doi.org/10.15690/vramn.v69i5-6.1054.
15. Bronisz E., Kurkowska-Jastrzębska I. Matrix metalloproteinase 9 in epilepsy: the role of neuroinflammation in seizure development. Mediators Inflamm. 2016; 2016: 7369020. https://doi.org/10.1155/2016/7369020.
16. Croll S.D., Ransohoff R.M., Cai N., et al. VEGF-mediated inflammation precedes angiogenesis in adult brain. Exp Neurol. 2004; 187 (2): 388–402. https://doi.org/10.1016/j.expneurol.2004.02.010.
17. Tröscher A.R., Gruber J., Wagner J.N., et al. Inflammation mediated epileptogenesis as possible mechanism underlying ischemic post-stroke epilepsy. Front Aging Neurosci. 2021; 13: 781174. https://doi.org/10.3389/fnagi.2021.781174.
18. Rakhimbaeva G.S., Rashidova N.S. Neuron-specific enolase in blood serum as a diagnostic marker of epilepsy. Meždunarodnyj nevrologičeskij žurnal / International Neurological Journal. 2011; 2: 123–8 (in Russ.).
19. Bai X., Zhang L., Li H., et al. A cross-sectional study of early-onset epilepsy of intracerebral hemorrhage and construction of a risk prediction model. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022; 34 (12): 1273–9 (in Chinese). https://doi.org/10.3760/cma.j.cn121430-20221008-00878.
20. Eriksson H., Banote R.K., Larsson D., et al. Brain injury markers in new-onset seizures in adults: a pilot study. Seizure. 2021; 92: 62–7. https://doi.org/10.1016/j.seizure.2021.08.012.
21. Ogaki A., Ikegaya Y., Koyama R. Vascular abnormalities and the role of vascular endothelial growth factor in the epileptic brain. Front Pharmacol. 2020; 11: 20. https://doi.org/10.3389/fphar.2020.00020.
22. Rigau V., Morin M., Rousset M.C., et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007; 130 (Pt 7): 1942–56. https://doi.org/10.1093/brain/awm118.
23. Zabrodskaya Y., Paramonova N., Litovchenko A., et al. Neuroinflammatory dysfunction of the blood-brain barrier and basement membrane dysplasia play a role in the development of drug-resistant epilepsy. Int J Mol Sci. 2023; 24 (16): 12689. https://doi.org/10.3390/ijms241612689.
24. Rasulova Kh.A. Angiogenesis in normal and pathological conditions. The role of vascular endothelial growth factor. Neurology. 2016; 3: 47–9 (in Russ.).
25. Park S.J., Park S.H. Systemic expression of vascular endothelial growth factor in patients with cerebral cavernous malformation treated by stereotactic radiosurgery. J Korean Neurosurg Soc. 2016; 59 (5): 442–8. https://doi.org/10.3340/jkns.2016.59.5.442.
26. Li P., Zhang L., Chen D., et al. Focal neurons: another source of vascular endothelial growth factor in brain arteriovenous malformation tissues? Neurol Res. 2018; 40 (2): 122–9. https://doi.org/10.1080/01616412.2017.1405574.
27. Guo D., Zhang B., Han L., et al. Cerebral vascular and blood brain-barrier abnormalities in a mouse model of epilepsy and tuberous sclerosis complex. Epilepsia. 2024; 65 (2): 483–96. https://doi.org/10.1111/epi.17848.
28. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008; 25 (5): 457–507. https://doi.org/10.1159/000131083.
29. Fisher R.S., Acevedo C., Arzimanoglou A., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014; 55 (4): 475–82. https://doi.org/10.1111/epi.12550.
30. Fisher R.S., Cross J.H., D'Souza C., et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017; 58 (4): 531–42. https://doi.org/10.1111/epi.13671.
31. Galovic M., Döhler N., Erdélyi-Canavese B., et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 2018; 17 (2): 143–52. https://doi.org/10.1016/S1474-4422(17)30404-0.
32. Beghi E., D'Alessandro R., Beretta S., et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology. 2011; 77 (20): 1785–93. https://doi.org/10.1212/WNL.0b013e3182364878.
33. Holtkamp M., Beghi E., Benninger F., et al. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur Stroke J. 2017; 2 (2): 103–15. https://doi.org/10.1177/2396987317705536.
34. Lühdorf K., Jensen L.K., Plesner A.M. Epilepsy in the elderly: incidence, social function, and disability. Epilepsia. 1986; 27 (2): 135–41. https://doi.org/10.1111/j.1528-1157.1986.tb03516.x.
35. Rundgren M., Karlsson T., Nielsen N., et al. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009; 80 (7): 784–9. https://doi.org/10.1016/j.resuscitation.2009.03.025.
36. Kaca-Oryńska M., Tomasiuk R., Friedman A. Neuron-specific enolase and S 100B protein as predictors of outcome in ischaemic stroke. Neurol Neurochir Pol. 2010; 44 (5): 459–63. https://doi.org/10.1016/s0028-3843(14)60136-5.
37. Kurakina А.S., Semenova T.N., Guzanova E.V., et al. Prognostic value of investigating neuron-specific enolase in patients with ischemic stroke. Sovremennye tehnologii v medicine / Modern Technologies in Medicine. 2021; 13 (2): 68–72. https://doi.org/10.17691/stm2021.13.2.08.
About the Authors
G. S. RakhimbaevaUzbekistan
Gulnora S. Rakhimbaeva - Dr. Sci. Med., Prof.
2 Shifokorlar Str., Tashkent 100109
Scopus Author ID 37122562300
D. S. Sobirova
Uzbekistan
Donokhon S. Sobirova.
2 Shifokorlar Str., Tashkent 100109; 24 Gayrati Str., Tashkent 100099
Review
For citations:
Rakhimbaeva G.S., Sobirova D.S. Сlinical and neuroimmunological correlations in post-stroke epilepsy illustrated by analyzing serum neuron-specific enolase and vascular endothelial growth factor. Epilepsy and paroxysmal conditions. 2024;16(4):316-326. https://doi.org/10.17749/2077-8333/epi.par.con.2024.205
JATS XML

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.







































