Scroll to:
Role of neuropeptide Y in development of valproate-induced eating behaviour disorder
https://doi.org/10.17749/2077-8333/epi.par.con.2024.207
Abstract
Background. Eating behaviour disorder (EBD) induced by valproic acid (VPA) is one of the components of the pathogenesis of a serious complication of therapy with VPA and its salts such as VPA-induced metabolic syndrome (MetS). About 20% of patients receiving VPA have weight gain, which is also a consequence of altered eating behaviour in such patients. Substances such as neuropeptide Y (NPY), leptin, orexin and ghrelin are involved in the regulation of eating behaviour. NPY has received special attention in recent years because it is one of the most potent brain orexigenic peptides and its expression level directly affects the quantity and quality of food intake. NPY overexpression is associated with EBD, food preferences, obesity, and MetS.
Objective: to review preclinical and clinical studies of NPY role as a potential sensitive and specific serum biomarker of VPA-EBD, secondary weight gain and VPA-MetS development in children and adults with epilepsy.
Material and methods. We analyzed Russian and foreign publications submitted to eLibrary, PubMed/MEDLINE, Scopus, and Google Scholar databases between 2014 and 2024. The full-text articles in Russian and English (original studies, systematic reviews, meta-analyses, Cochrane reviews, and clinical cases) were analyzed. After the selection procedure, 53 out of 1105 publications retrieved by query keywords were included in the analysis.
Results. VPA-EDB refers to multifactorial diseases, requiring to take into account the additive contribution of external (food education, eating habits of the patient and family members) and internal (key hormones and neuropeptides regulating appetite and food preferences, the dose and duration of VPA intake, the metabolic rate of VPA) factors while assessing a risk of its development. NPY-associated VPA-EDB affects dietary preferences in favor of high-calorie food and beverages, increases the frequency of meals, the risks of insulin resistance, hyperglycemia as one of the major domains in MetS pathogenesis.
Conclusion. VPA-EBD requires timely diagnosis, as it can cause VPA-MetS. NPY is an important biomarker of VPA-EBD, because recent studies have convincingly demonstrated that this neuropeptide is involved in the regulation of eating behavior in patients with epilepsy.
Keywords
For citations:
Shnayder N.A., Grechkina V.V., Kissin M.Ya., Dmitrenko D.V., Nasyrova R.F. Role of neuropeptide Y in development of valproate-induced eating behaviour disorder. Epilepsy and paroxysmal conditions. 2024;16(4):349-361. https://doi.org/10.17749/2077-8333/epi.par.con.2024.207
INTRODUCTION / ВВЕДЕНИЕ
“Eating behaviour” – is a broad term that includes food choices and motivation, eating practices, diets and eating-related problems (obesity, eating behaviour disorders (EBDs) and feeding disorders) [1]. Multiple genetic, physiological, psychological, cultural, social and situational factors influence the formation of eating behaviour [2].
Eating behaviour in humans is not stereotyped, determined not only by the need to compensate for acute changes in energy status. It is evident that emotional [3][4], cognitive [5][6] and cultural factors [7][8] as well as the intake of some medications [9] play an important role in the initiation and termination of the act of eating. Although a negative energy balance is sufficient, it is not necessary to start a meal.
The different types of eating behaviour control (homeostatic, hedonic and cognitive) are closely intertwined in real life. Their separation into discrete mechanisms is mostly supported by theoretical arguments rather than empirical evidence [10]. Hunger and satiety are at the crossroads of this complex interplay between metabolic and non-metabolic factors that regulate eating behaviour in children and adults. The energy balance in the human body is constantly controlled by the brain through a variety of endocrine and neural mechanisms that include long- and short-term signals of changes in energy stores (energy “currency”). Against this dynamic background, which stimulates the individual to decide whether to start eating or not, to stop eating or not, information from the external environment – sensory (sight, smell or taste of food) or social (the presence of a planned lunch break) – can act as triggers [10].
EBD associated with medication intake, including antiepileptic drugs (AEDs), are still understudied, and the development of abdominal (central) obesity as a key component of AEDs-induced metabolic syndrome (MetS) [11]. Unfortunately, most epileptologists (neurologists and psychiatrists) do not diagnose EBD on the background of long-term AED taking in children and adults with epilepsy, which leads to the loss of the therapeutic window when prevention of AED-induced MetS development is most effective.
AEDs of different pharmacological groups may have variable adverse effects on changes in eating behaviour, increase the risk of obesity and MetS in patients with epilepsy. AEDs with the highest risk of these adverse reactions include valproic acid (VPA) and its compounds [11][12]. Despite the large number of observational studies, evidence that VPA significantly increases the risk of VPA-induced EBD (VPA-EBD), obesity and MetS (VPA-MetS) [11], VPA-EBD biomarkers are still poorly understood. In recent years, there has been an increasing interest of researchers in studying the role of brain neuropeptides involved in the regulation of eating behaviour in healthy individuals and epilepsy patients, including patients on long-term VPA treatment.
Objective: to review preclinical and clinical studies on NPY role as a potential sensitive and specific serum biomarker of VPA-EBD, secondary weight gain and VPA-MetS development in children and adults with epilepsy.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
Search strategy / Стратегия поиска
Full-text publications were searched in eLibrary, PubMed/MEDLINE, Scopus, Google Scholar databases using keywords in Russian and English: “neuropeptide Y”, “eating behaviour”, “eating disorder”, “metabolic syndrome”, “valproic acid”, “valproate”, “valproate”, “adverse reaction”, “valproate-induced adverse reactions”, ‘valproate-induced eating disorder’, “neuropeptide Y”, “eating behaviour”, “eating disorder”, “metabolic syndrome”, “valproic acid”, “valproates”, “adverse reaction”, “valproate-induced adverse reactions”, “valproate-induced eating disorder”. The search was performed over a one-year period (from 10 July 2024 to 20 July 2024).
Placebo-controlled and cross-sectional studies, case-control studies, case-control studies, case studies, systematic reviews, meta-analyses and Cochrane reviews entered into databases from 2014 to 2024 were considered. In addition, earlier publications of historical interest are included in the review.
Selection of publications / Отбор публикаций
The selection was done by double independent review. Articles in which data were not statistically significant or were questionable, as well as duplicate texts were excluded. A total of 1105 publications were retrieved using keywords, and after the selection procedure, 53 articles that met the purpose of this review were included in the analysis.
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Risk factors for altering eating behavior in epilepsy patients / Факторы риска изменения пищевого поведения у пациентов с эпилепсией
Environmental risk factors
Epilepsy is not only a common socially significant neurological disease but also a social problem for children/teenagers and their parents as well as adult patients.
The eating behaviour in patients receiving VPA may be influenced by external risk factors such as eating patterns in family members. Parents of children and teenagers with epilepsy, as well as family members of adult patients, fear the unpredictability of epileptic seizures, worry about the physical and mental health of their family members (bullying among peers and probands), learning difficulties, reduced ability to self-care and independence from outside help in the future [13]. In addition, the eating behaviour of parents (family members) may contribute to the formation of eating habits in their children, which may also influence changes in body weight of the latter [13]. Parenting behaviour is a shared behavioural structure that defines the emotional context in which parents and children interact and reflects the emotional climate in which children with epilepsy are brought up [14]. Three main methods can be used in the nutritional education of children and teenagers with epilepsy [15]: structure, coercive control, support for autonomy.
The structure of food education is related to rules and boundaries and includes eating and snacking routines, food availability and accessibility factors. Coercive control refers to methods associated with negative attitudes, including restraint, forced eating, threats and rewards. In contrast, supporting autonomy for patients with epilepsy involves the use of encouragement and nutrition education methods and is associated with healthier food consumption [15].
A variety of meals at home, regular exposure of the child with epilepsy to new and familiar foods, verbal encouragement and parental modelling of meals are successful parenting strategies for increasing compliance with food rules. However, parental methods such as controlling food intake, suppressing consumption, restricting foods and using rewards can have a negative impact on children's and teenagers' eating behaviour, leading to reduced continuity of eating habits and aversion to foods children should eat and avoid eating [14]. Unfortunately, the negative additive effect of inadequate nutritional education of patients with childhood epilepsy and EBD is rarely considered when assessing the risk of developing VPA-induced obesity and VPA-MetS.
Hormonal risk factors
NPY is a 36 amino acid protein that belongs to the pancreatic polypeptide family and is involved in a variety of physiological and homeostatic processes in both the central (CNS) and peripheral nervous system (PNS), including the regulation of cognitive and executive functions, nutritional behaviour and energy balance, sex hormone secretion, modulation of cardiovascular and neuroendocrine function, stress response, emotional behaviour, neuronal excitability, epileptogenesis, and adherence to ethanol consumption [16][17]. NPY was first isolated in 1982 by K. Tatemoto et al. [18] from the pig hypothalamus and immediately attracted the close attention of researchers from various fields of neuroscience [19].
NPY is one of the most abundant neuropeptides in the CNS, where it is predominantly expressed in the cortex, hippocampus, hindbrain and hypothalamus [16]. NPY mediates its action through neuropeptide Y receptors, mainly of the Y1, Y2, Y4 and Y5 types [20]. NPY receptors are widely present in the CNS and PNS, as well as in postganglionic sympathetic fibres, adrenal glands, megakaryocytes and platelets [21]. Y1 and Y5 type receptors play an important role in increasing appetite, while Y2 and Y4 type receptors are probably involved in appetite suppression (the appearance of a feeling of satiety). NPY has been shown to exert its effects through modulation of guanidine nucleotide (G) subtype activity [22].
The NPY system involved in the regulation of eating behaviour is mainly located in the hypothalamus, including the arcuate nucleus (ARC), where NPY and agouti-related peptide (AgRP) are synthesized. AgRP is involved in increasing appetite and reducing metabolism and energy expenditure. It is synthesizes in the paraventricular nucleus (PVN), dorsomedial nucleus (DMN) and ventromedial nucleus (VMN) in the prefrontal region also [16, 23]. ARC neurons direct their axons to the PVN as well as to the DMN and medial preoptic area [16].
The biological effects of NPY have been investigated in mouse models, and it has been shown to shorten the time to the start of the next meal. In contrast, the absence of NPY in NPY gene knockout mice was associated with a marked delay in the onset of food intake. NPY also delays the feeling of satiety, resulting in an increase in portion size, time spent eating, and meal duration. These effects of NPY were independent of food type, as they were observed when experimental animals were fed both solid dry food and sweetened milk [16]. Also, NPY is involved in eating behaviour, including the regulation of motivation to eat; this effect of NPY is comparable to 36–48 h fasts [16]. Intracerebral injection of NPY alters motivation for the quality components of the food ingested in addition to increasing the quantity of food consumed [16][24].
The type of carbohydrate plays an important role in the stimulatory orexigenic effect of NPY. When experimental animals can choose between a high-carbohydrate and high-fat diet, they prefer the first one [16, 25]. This choice may be due to a change in the palatability of the food consumed due to the increased sweetness of sucrose or polycose diets compared to corn starch. This is supported by an experiment in which various solutions sweetened with sucrose or saccharin were tested on satiated rats: NPY increased consumption of various sucrose solutions (2–10%) as well as the preferred saccharin solution [16][26].
One important extrinsic factor affecting the level of NPY expression in the hypothalamus is starvation. When rats are deprived of food for 24–96 h, NPY expression in ARC is significantly increased [16]. Also, chronic fasting increases NPY expression in the ARC, but additionally increases NPY expression in the hindbrain, which is not observed after a short period of fasting [27]. In parallel, an increase in NPY expression in the PVN after chronic fasting was observed. Resumption of normal feeding rapidly returns NPY levels in ARC to baseline values, but in PVN NPY expression remains elevated even after 6 h of resumption of feeding, which may be due to the fact that the body weight of rats during this period has not yet returned to normal (on average, the weight of an experimental animal normalizes after about 24 h) [16][27].
Hypoglycemia is one of the factors regulating NPY expression in the CNS [28]. Glucose-sensitive neurons containing NPY are present in the ARC and may play an integrative role in the regulation of eating behaviour and energy balance [16]. Glucoprivation induced by injection of the metabolic blocker 2-deoxyglucose (2DG) leads to increased appetite through activation of hypothalamic NPY neurons and increased NPY expression in the ARC and hindbrain. Moreover, appetite induced by 2DG administration is reduced after administration of antibodies to NPY in the PVN and knockout of the NPY gene [16]. An important role of glucose availability in the regulation of NPY expression in the CNS has been confirmed [28]. Thus, after intravenous glucose injection, stimulation of food intake by NPY is less than after intravenous injection of physiological sodium chloride solution [16].
NPY-induced EBD also affects plasma insulin levels [29], which are reduced during fasting and can also be suppressed by the administration of streptozocin, which induces pancreatic β-cell death. In an animal model of diabetes mellitus, it has been shown that NPY sensitivity is decreased in the CNS, whereas NPY expression and release are increased in the hypothalamus and the ARC-PVN. In addition, the number of receptors for NPY is reduced in streptozocin rats. NPY expression normalizes after insulin administration [16].
Important endogenous factors involved in NPY-induced EBD include glucocorticoids and leptin [30]. Leptin receptors are expressed in NPY neurons localized in the ARC. Leptin expression in adipose tissue and its plasma levels are reduced during fasting, resulting in upregulation of the NPY system with increased mRNA expression and NPY synthesis in the ARC, enhanced NPY release in the PVN. On the other hand, chronic intracerebral administration of NPY leads to an increase in leptin mRNA in adipocytes [16].
In obesity, it is very rare to find a mutation of the LEP gene encoding leptin or the NPY gene alone, as central obesity and MetS are multifactorial diseases in which the environment and nutritional conditions of the patient with epilepsy play a major role. Multiple studies have shown that central obesity can be caused by eating unbalanced diets high in carbohydrates and fats or high-calorie diets [16].
It is known, that central and peripheral regulation of body weight (in both children and adults) involves a variety of neuropeptides and cytokines, including adiponectin, leptin, ghrelin, visfatin, and NPY. At the same time, adiponectin and leptin are among potential regulators of glucose and lipid metabolism, which are altered against the background of long-term VPA therapy [31], and leptin and ghrelin can activate receptors in ARC through modulation of gamma-aminobutyric acid effects, which leads to EBD and disturbance of energy homeostasis [32][33]. Changes in leptin levels in children and adults with VPA-induced weight gain have been shown in many studies [32][34–36]. In most studies, this adverse reaction was associated with increased appetite and abnormal thirst that was quenched by sweet or high-energy drinks, and several studies demonstrated that VPA-induced weight gain in patients with epilepsy can be reversible with behavioural psychotherapy without discontinuation of VPA intake [36].
VPA can directly inhibit the expression of the ADIPOQ gene encoding adiponectin of differentiated adipocytes [37], but clinical data on whether VPA alters the expression of this gene in people with epilepsy are still insufficient, although changes in serum levels of adiponectin and insulin resistance have been found in patients with bipolar affective disorder on the background of therapy with VPA [38][39].
Visfatin is a hormone that exerts insulin-mimetic action and reduces blood glucose levels. In turn, hyperglycemia can stimulate food intake by affecting glucose-sensitive neurons in the medial hypothalamus and subsequently reducing the production of efferent inhibitor visfatin in the lateral hypothalamus [40].
In addition. to these neuropeptides, candidate hormones associated with VPA-EBD, weight gain and VPA-MetS include ghrelin, glucagon-like peptide 1 type (GLP-1) and YY peptide [11]. Increased ghrelin expression is known to stimulate appetite and increase food intake [41]. In contrast, GLP-1 and peptide YY suppress appetite and reduce the number of meals eaten [42]. However, the effect of changes in serum ghrelin levels in epileptic patients receiving VPA on their weight change has not yet been proven, and the effects of VPA on serum levels of GLP-1 and YY peptide continue to be studied [43].
VPA may also alter the microstructure of food intake (e.g., altering the rate of food ingestion), which affects the rate of production and level of expression of satiety hormones in the CNS. It has been shown that gastric distension can activate GLP-1 synthesis [44]. The gradual increase in portions in patients with VPA-EBD can be modified by inhibition of this peptide. Finally, long-term taking of VPA may cause the bodies of patients with epilepsy to lose the ability to recognize the satiety signals associated with the effects of satiety hormones, rendering them ineffective [32].
NPY AS BIOMARKER OF VPA-EBD / NPY КАК БИОМАРКЕР ВК-РПП
VPA is a first-generation AEDs prescribed to children, teenagers and adults with epilepsy, although its mechanism of action remains poorly understood [45]. The widespread use of VPA and its compounds (valproates) in neurology and psychiatry can lead to the development of serious adverse reactions (especially in long-term therapy), a group of which includes VPA-MetS [11].
The mechanisms by which VPA increases body weight are not yet fully understood. Possible pathogenesis pathways for the development of VPA-induced obesity and VPA-MetS may include hyperglycemia, insulin resistance, and the effect of VPA on the balance between energy intake and energy expenditure. Changes in energy intake or expenditure are likely related to the effects of VPA on biological mechanisms that are involved in altering the metabolism of various hormones and neuropeptides, including NPY [43]. The ability of VPA and its active metabolites [45] to influence hypothalamic neurons, namely NPY expression, may be one of the key links in the pathogenesis of VPA-MetS development in general and VPA-induced weight gain in particular, since VPA-induced NPY hyperexpression increases appetite and cravings for foods high in carbohydrates and fat in epileptic patients.
The mechanism of action of VPA may determine changes in the activity of certain transcription factors that regulate the expression of key neuropeptides, including NPY [46]. Although chronic VPA therapy did not alter the release of NPY in the PVN, which plays a key role in the regulation of eating behaviour, the expression of NPY or its receptors may be altered in other hypothalamic nuclei associated with EBD, but this mechanism needs further investigation [47]
In the study of C.K. Martin et al. (2008), there are reported an increase in hunger in patients receiving VPA, as opposed to a control group of naive patients, which was accompanied by increased cravings for high-fat foods, which contributed to weight gain in the VPA group [44]. Systematic review by M.B. Torlasco and M.A. Estrin (2023) [48] demonstrated the relationship between VPA therapy, increased NPY expression, and increased body weight.
A. Cansu et al. [49] in a study involving 18 children aged 3.4 to 15.8 years and a group of 18 healthy children of comparable sex and age demonstrated that after 18 months of treatment, the average weight gain in the VPA group was 2.3 kg more than in children in the control group. The blood levels of insulin, leptin, NPY and galanin increased statistically significantly in the VPA group, while in contrast, ghrelin levels decreased compared to the control group [49].
Study by K. Aydin et al. [35] included 20 patients (10 boys and 10 girls) with newly diagnosed epilepsy aged 4 to 12 years. The authors measured body mass index (BMI), serum levels of NPY, glucose, insulin, cortisol and leptin before starting VPA and 3 and 6 months after starting therapy. At the end of 3 months of therapy, BMI and serum levels of insulin and NPY significantly increased and glucose levels decreased. After 6 months of VPA taking, an increase in serum concentrations of leptin and cortisol was observed. In addition, leptin was higher in girls than in boys, and serum glucose, insulin, cortisol, and NPY levels were independent of the patient's' gender. Importantly, the serum VPA content in the observed children with epilepsy was within the reference therapeutic corridor (83.94±21.85 µg/ml). No correlation was found between blood VPA levels and BMI and serum concentrations of glucose, insulin, cortisol, leptin and NPY. Serum biomarkers of liver function in patients during VPA treatment were normal [35].
The study by H. Tokgoz et al. [50] included 20 children (8 girls and 12 boys), whose mean age was 8.75±1.62 years. The patients received VPA monotherapy, of which 10 children had genetic focal epilepsy and 10 had genetic generalized epilepsy. The authors assessed weight, height, BMI, serum levels of glucose, insulin, leptin, NPY, ghrelin, adiponectin and cortisol before treatment and 6 and 12 months after treatment initiation. A significant increase in BMI and height at the end of the 6th and 12th months of follow-up was shown: BMI increased significantly at the study endpoints, with a higher BMI after 12 months of VPA monotherapy than after 6 months of follow-up. Serum glucose concentrations did not change statistically significantly, but insulin levels tended to steadily increase at the end of the 6th and 12th months. Compared to baseline, serum levels of NPY and leptin significantly increased on long-term VPA monotherapy. Cortisol content increased in the first 6 months but not after 12 months. However, ghrelin and adiponectin levels did not change statistically significantly during the entire observation period compared to baseline values. There were no statistically significant changes in plasma concentrations of classical markers of metabolic syndrome (total cholesterol, triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL)) in any of the measurements. Also, there were no significant differences between the studied parameters in boys and girls and depending on the form of epilepsy [50].
In a study of the effects of VPA and topiramate (TPM) on insulin, leptin, NPY and ghrelin levels, 45 children with epilepsy were included, of whom 25 received VPA and 23 received TPM. Participants ranged in age from 6 to 15.5 years. The duration of VPA or TPM monotherapy was at least 6 months. The control group included 25 healthy children. Blood samples were taken in the main and control groups after fasting for at least 10–12 h and 1 and 2 h after a meal. Age, height, weight and BMI at the beginning of the study were comparable in the main and control groups. Significant weight gain was observed throughout the treatment in the VPA group compared to the TPM group. High fasting serum insulin and post-meal insulin levels were noted in the VPA group. Leptin and NPY concentrations in the VPA group were also statistically significantly higher than in the TPM and control groups. No significant differences were found in serum ghrelin content in the main group compared to the control group [51].
In addition to investigating the role of VPA in altering NPY levels and increasing body weight in patients with epilepsy it has been shown that VPA has a clinically significant negative effect on the incidence of VPA-EBD and obesity, especially in female patients at pre-pubertal (17–19 years) and post-pubertal (>19 years) ages [32]. At post-pubertal age, weight gain between 1 and 10 kg was recorded in 57% of cases on VPA monotherapy compared to 25% of cases in patients receiving lamotrigine monotherapy [32]. This negative effect of VPA has been linked to both changes in eating behaviour in female patients with epilepsy and the development of insulin resistance, including an increase in the HOMA-IR index (>2.5) at a mean of 3.2 years from the start of therapy [32]. Increased serum insulin concentration and increasing severity of obesity in adult patients with epilepsy were associated with minor changes in serum lipid profile, including VPA-induced changes in other MetS biomarkers: decreased HDL levels and increased TG levels [32].
VPA-EBD DIAGNOSTICS / ДИАГНОСТИКА ВК-РПП
VPA-EBD in patients with epilepsy may be associated with modifiable and non-modifiable risk factors (Table 1) [33], is severe and often chronic, leading to the development of VPA-MetS, especially if not properly managed. Timely identification of VPA-EBD risk factors can help to understand patterns of psychosocial, biological and genetic predisposition to disordered eating in children, teenagers and adults with epilepsy, even in the absence of any overt weight or nutritional problems. Increasing epileptologists' awareness of VPA-EBD and VPA-MetS is an important way to improve the safety of AEDs use in epileptology.
EBD associated with overeating is characterized by recurrent episodes of overeating, i.e. episodes of eating behaviour occurring in a discrete period of time (≤2 h) and involving the consumption of an amount of food that is definitely greater than most people would consume in similar circumstances). Other key features of EBD associated with overeating are feelings of lack of control over eating during episodes of overeating, significant psychological distress (e.g., shame, guilt) due to overeating, and lack of recurrent maladaptive compensatory behaviour [52]. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM) 4th and 5th revisions, the diagnosis of overeating-related EBD is based on five criteria (Table 2). [52].
The 10-point Hunger and Satiety Scale [53, 54] (Fig. 1), the Intuitive Eating Chart (Table 3) and the Healthy Eating Behaviour Checklist1 can be used in the practice of an epileptologist for screening and management of patients with VPA-EBD [53][54].
Table 1. Modifiable and non-modifiable risk factors for valproate-induced eating behaviour disorder (adapted with changes from [33])
Таблица 1. Модифицируемые и немодифицируемые факторы риска вальпроат-индуцированного расстройства пищевого поведения (адаптировано с изменениями из [33])
Modifiable risk factors / | Non-modifiable risk factors / |
Age of onset for VPA or its compounds therapy / Возраст начала терапии ВК или ее соединениями | Age (children and adolescents) / Возраст (дети и подростки) |
High-dose VPA or its compounds therapy / | Sex (female) / Пол (женский) |
Long-term VPA or its compounds therapy / | Race (ethnicity) / Раса (этнос) |
Polytherapy (simultaneous use of ≥2 AEDs) / Политерапия (одновременный прием ≥2 ПЭП) | Genetic predisposition (gene polymorphisms: NPY, GHRL, LEP, MC4R, DRD2, DRD4, ANKK1, DAT1, SLC6A4, OPRM1, BNDF, FTO, GR, NTRK2, COMT, ESR1, CNR1, etc.) / Генетическая предрасположенность (полиморфизмы генов NPY, GHRL, LEP, MC4R, DRD2, DRD4, ANKK1, DAT1, SLC6A4, OPRM1, BNDF, FTO, GR, NTRK2, COMT, ESR1, CNR1 и др.) |
Polypragmasy (simultaneous use of ≥5 drugs) / Полипрагмазия (одновременный прием ≥5 ЛС) | In utero exposure to high cortisol levels due to maternal stress / Внутриутробное воздействие высокого уровня кортизола в результате материнского стресса |
Nutrition (frequency of meals, portion sizes, food preferences) / Питание (кратность питания, объем порций, пищевые предпочтения) | Раннее половое созревание / Precocious puberty |
Low physical activity and prolonged bed rest / | Retarded intellectual development, cognitive impairment, low education level / Задержка интеллектуального развития, когнитивные расстройства, низкий уровень образования |
Gut microbiota (dysbiosis of intestinal microorganisms) / Микробиота кишечника (дисбиоз кишечных микроорганизмов) | Personality traits of epilepsy patient (perfectionism, obsession, impulsiveness) / Личностные особенности больного эпилепсией (перфекционизм, одержимость, импульсивность) |
Relationships between a patient with epilepsy and family members (family members' opinion that a patient is underweight, pressure from them regarding a patient's diet) / Взаимоотношения больного эпилепсией с членами семьи (мнение членов семьи о том, что у пациента низкий вес, давление с их стороны в отношении питания пациента) | Endocrine comorbidities (diabetes mellitus, hypothyroidism, hyperthyroidism) / Коморбидные заболевания эндокринной системы (сахарный диабет, гипотиреоз, гипертиреоз) |
Socioeconomic status (food insecurity leading to overeating, family eating habits leading to overeating, overprotectiveness by family members) / Социально-экономический статус (отсутствие продуктовой безопасности, приводящее к перееданию, привычки питания в семье, приводящие к перееданию, гиперопека со стороны членов семьи) | Mental comorbidities (compulsive-impulsive disorder, post-traumatic stress disorder, social anxiety disorder, borderline personality disorder, bipolar disorder, depressive disorder) / Коморбидные психические расстройства (компульсивно-импульсивное расстройство, посттравматическое стрессовое расстройство, социальное тревожное расстройство, пограничное расстройство личности, биполярное расстройство, депрессивное расстройство) |
Emotional abuse (experience of childhood trauma and abuse) / Эмоциональное насилие (опыт детских травм и жестокого обращения) | Autoimmune comorbidities (gastrointestinal tract inflammatory diseases) / Коморбидные аутоиммунные заболевания (воспалительные заболевания желудочно-кишечного тракта) |
Note. VPA – valproic acid; AEDs – antiepileptic drugs.
Примечание. ВК – вальпроевая кислота; ПЭП – противоэпилептический препарат; ЛС – лекарственное средство.
Table 2. Diagnostic criteria for eating behaviour disorder associated with overeating [52]
Таблица 2. Критерии диагностики расстройства пищевого поведения, связанного с перееданием [52]
Criteria / Критерий | Characteristics / Характеристики |
Criterion 1 /Критерий 1 | Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following /Повторяющиеся эпизоды переедания. Эпизод переедания характеризуется следующим: а) eating in a discrete period of time (e.g., within any 2-hour period), an amount of food that is definitely larger than most people would eat in a similar period of time under similar circumstances / еда в дискретный период времени (например, в течение любого 2-часового периода); количество пищи, которое больше, чем большинство людей ели бы в аналогичный период времени при аналогичных обстоятельствах; b) the sense of lack of control over eating during the episode (e.g., a feeling that one cannot stop eating or control what or how much one is eating) / чувство отсутствия контроля над едой во время эпизода (например, ощущение, что нельзя перестать есть или контролировать, что или сколько пациент ест) |
Criterion 2 /Критерий 2 | Binge-eating episodes are associated with three (or more) of the following / Эпизоды переедания связаны с тремя (или более) следующими критериями: а) eating much more rapidly than normal / употребление пищи гораздо быстрее, чем обычно; b) eating until feeling uncomfortably full / употребление пищи до тех пор, пока пациент не почувствует себя некомфортно сытым (переедание); c) eating large amounts of food when not feeling physically hungry / употребление большого количества пищи, когда пациент не чувствует физического голода; d) eating alone because of being embarrassed by how much one is eating / употребление пищи в одиночестве из-за того, что пациенту стыдно за то, сколько он ест; e) feeling disgusted with oneself, depressed, or very guilty after overeating / чувство отвращения к себе, депрессия или чувство вины после переедания |
Criterion 3 /Критерий 3 | Marked distress regarding binge eating is present / Отмечаются неприятные чувства (стыд, грусть, вина и т.д.), связанные с перееданием |
Criterion 4 /Критерий 4 | The binge eating occurs, on average / В среднем происходит переедание: а) at least 2 days a week for 6 months (DSM-4 frequency and duration criteria) / не менее 2 дней в неделю в течение 6 мес (критерии частоты и продолжительности DSM-4); b) at least 1 day a week for 3 months (DSM-5 frequency and duration criteria) / не менее 1 дня в неделю в течение 3 мес (критерии частоты и продолжительности DSM-5) |
Criterion 5 /Критерий 5 | The binge eating is not associated with the regular use of inappropriate compensatory behavior (e.g., purging, fasting, excessive exercise) and does not occur exclusively during the course of anorexia nervosa or bulimia nervosa / Регулярное употребление пищи не связано с регулярным использованием ненадлежащего компенсаторного поведения (например, чистка, голодание, чрезмерные физические упражнения) и не происходит исключительно во время эпизода нервной анорексии или нервной булимии |
Severity grading /Оценка | DSM-4 does not include a binge-eating disorder severity grading scale / DSM-4 не включает Шкалу оценки тяжести расстройства пищевого поведения. Applicable to DSM-5 only, binge-eating disorder severity is graded as follows / Применимо только к DSM-5, тяжесть расстройства пищевого поведения оценивается следующим образом: – mild: 1 to 3 episodes per week / легкое: от 1 до 3 эпизодов в неделю; – moderate: 4 to 7 episodes per week / умеренное: от 4 до 7 эпизодов в неделю; – severe: 8 to 13 episodes per week / тяжелое: от 8 до 13 эпизодов в неделю; – extreme: 14 or more episodes per week / экстремальное: 14 и более эпизодов в неделю |
Note. DSM – Diagnostic and Statistical Manual of Mental Disorders.
Примечание. DSM (англ. Diagnostic and Statistical Manual of Mental Disorders) – Диагностическое и статистическое руководство по психическим расстройствам.
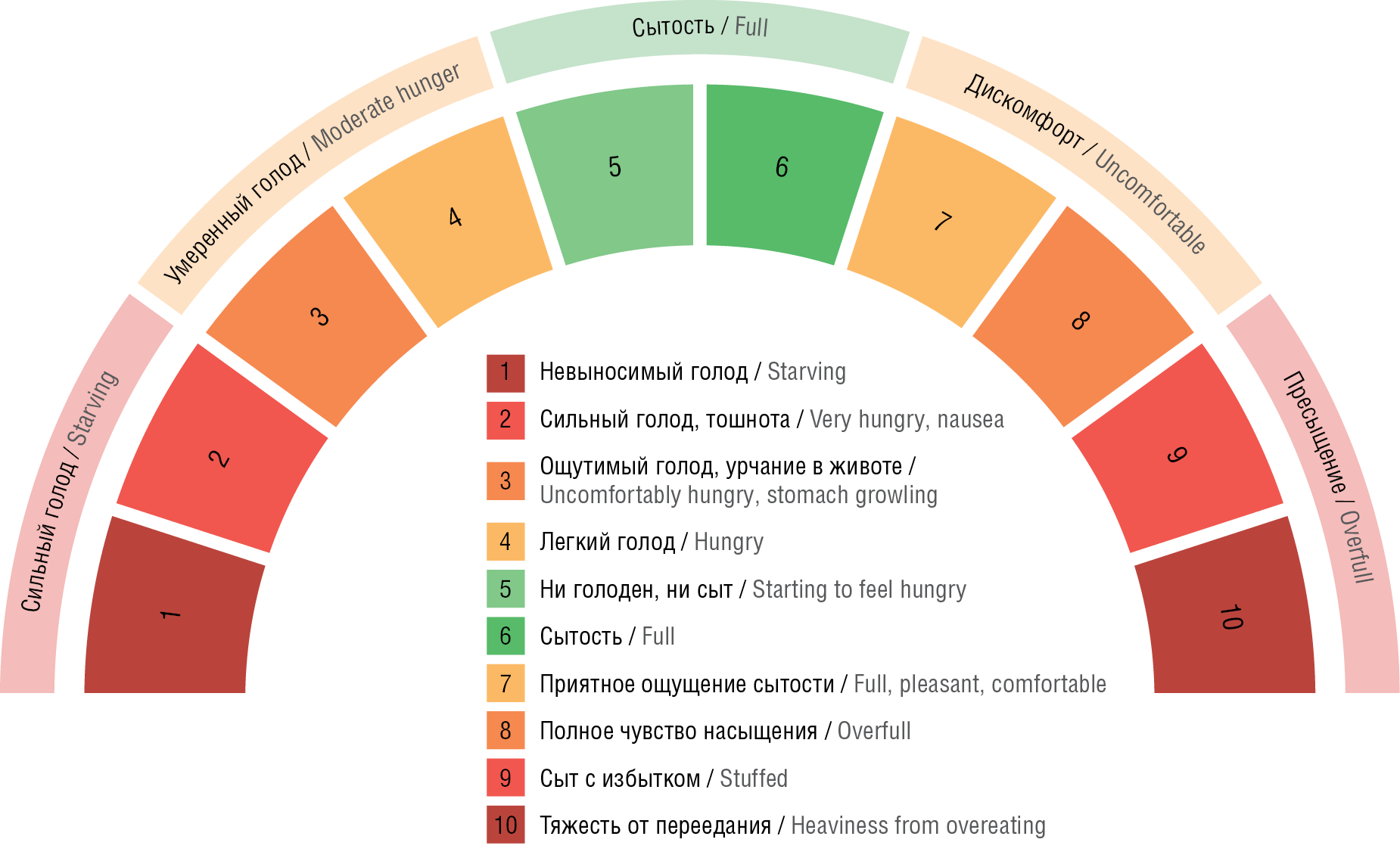
Figure 1. Hunger-Satiety Scale [53][54]
Рисунок 1. Шкала голода и насыщения (сытости) [53][54]
Table 3. Intuitive Eating Chart
Таблица 3. Таблица интуитивного питания
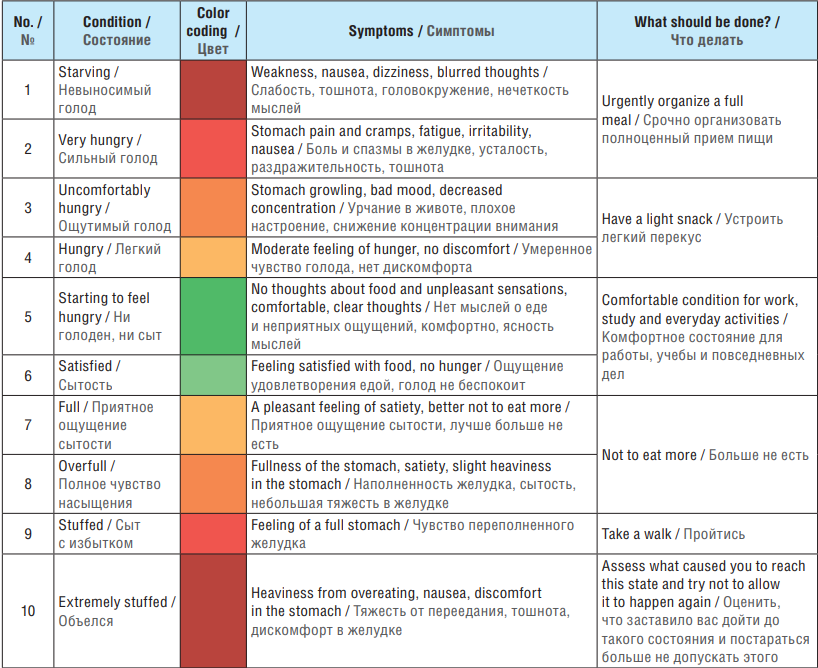
The importance of early diagnosis and timely correction of VPA-EBD in patients with epilepsy lies in predicting and reducing the risk of developing VPA-MetS. Known MetS criteria (according to the Expert Consensus on Interdisciplinary Approach to the Management, Diagnosis and Treatment of Patients with Metabolic Syndrome) in adults include [55]:
А) main feature: central (abdominal) type of obesity – waist circumference >80 cm in women and >94 cm in men;
B) additional criteria:
– arterial hypertension (blood pressure ≥140/90 mm Hg),
– elevated TG levels (≥1,7 mmol/l),
– decrease in HDL levels (<1,0 mmol/l in men; <1,2 mmol/l in women),
– increase in LDL levels >3,0 mmol/l,
– fasting hyperglycemia (fasting plasma glucose ≥6,1 mmol/l),
– impaired glucose tolerance (plasma glucose 2 h after glucose load within ≥7,8 and ≤11.1 mmol/l).
In turn, the MetS criteria according to Adult Treatment Panel III, as well as the American Heart Association and the National Heart, Lung, and Blood Institute include [56, 57]:
– waist circumference >102 cm for men, >88 cm for women2;
– blood pressure level ≥130/85 mm Hg;
– laboratory values HDL <1.04 mmol/l, TG ≥1.7 mmol/l, glucose ≥5.6 mmol/l.
In Expert Consensus on a Multidisciplinary Approach to the Management, Diagnosis and Treatment of Patients with Metabolic Syndrome [55] indicated that the presence of central obesity and two of the additional criteria (laboratory values and/or arterial hypertension) is a basis for diagnosing a patient with MetS. However, in the studies we analyzed, the authors did not take into account the criterion of abdominal obesity, which is the main component in the diagnosis of MetS in general and VPA-MetS in particular in patients with VPA-EBD. Instead, BMI was used, which is not included in either the Russian or foreign MetS criteria [58].
CONCLUSION / ЗАКЛЮЧЕНИЕ
The presented review demonstrates that VPA-EBD in patients with epilepsy requires timely diagnosis as it may cause VPA-MetS. It is important to establish good eating habits in children, teenagers and adults with epilepsy, which may increase their compliance to VPA therapy and reduce the likelihood of developing VPA-EBD, weight gain and VPA-MetS.
Along with the known clinical and laboratory criteria for the diagnosis of VPA-EBD and VPA-MetS, NPY is an important biomarker because studies in recent years have convincingly demonstrated that this neuropeptide is involved in the regulation of eating behaviour in epileptic patients. In particular, the studies we analyzed showed a relationship between VPA monotherapy, increased serum NPY levels and weight gain in patients with epilepsy. Further studies on the relationship of NPY as a biological marker of VPA-EBD and VPA-induced weight gain will improve the quality of early diagnosis and prevention of VPA-MetS in adults and children with epilepsy.
1. https://online.synchronize.ru/check-list.
2. For comparison: US Food and Drug Administration criteria: >94 cm for men, >80 cm for women.
References
1. LaCaille L. Eating behavior. In: Gellman M.D., Turner J.R. (Eds.) Encyclopedia of behavioral medicine. Springer; 2013: 641–2. https://doi.org/10.1007/978-1-4419-1005-9_1613.
2. Stubbs R.J. Controlling appetite and food intake by regulating eating frequency and timing. In: Gill T. (Ed.) Managing and preventing obesity: behavioural factors and dietary interventions. Woodhead Publishing; 2014: 149–65. https://doi.org/10.1533/9781782420996.3.149.
3. Reichenberger J., Schnepper R., Arend A.K., Blechert J. Emotional eating in healthy individuals and patients with an eating disorder: evidence from psychometric, experimental and naturalistic studies. Proc Nutr Soc. 2020; 79 (3): 290–9. https://doi.org/10.1017/S0029665120007004.
4. Ha O.R., Lim S.L. The role of emotion in eating behavior and decisions. Front Psychol. 2023; 14: 1265074. https://doi.org/10.3389/fpsyg.2023.1265074.
5. Aoun C., Nassar L., Soumi S., et al. The cognitive, behavioral, and emotional aspects of eating habits and association with impulsivity, chronotype, anxiety, and depression: a cross-sectional study. Front Behav Neurosci. 2019; 13: 204. https://doi.org/10.3389/fnbeh.2019;00204.
6. Kakoschke N., Aarts E., Verdejo-García A. The cognitive drivers of compulsive eating behavior. Front Behav Neurosci. 2019; 12: 338. https://doi.org/10.3389/fnbeh.2018.00338.
7. Becuţ A.G., Puerto K.L. Introduction. Food history and identity: food and eating practices as elements of cultural heritage, identity and social creativity. Int Rev Soc Res. 2017; 7 (1): 1–4. https://doi.org/10.1515/irsr-2017-0001.
8. Asamane E.A., Greig C.A., Aunger J.A., Thompson J.L. Perceptions and factors influencing eating behaviours and physical function in community-dwelling ethnically diverse older adults: a longitudinal qualitative study. Nutrients. 2019; 11 (6): 1224. https://doi.org/10.3390/nu11061224.
9. Marzola E., Musso M., Abbate-Daga G. Psychotropic drug-induced disordered eating behaviors. In: Manzato E., Cuzzolaro M., Donini M.L. (Eds.) Hidden and lesser-known disordered eating behaviors in medical and psychiatric conditions. Springer; 2021: 77–86. https://doi.org/10.1007/978-3-030-81174-7_8.
10. Del Parigi A. Chapter 20 – Neuroanatomical correlates of hunger and satiaty in lean and obese individuals. In: Dube L., Bechara A., Dagher A., et al. (Eds.) Obesity prevention: the role of brain and society on individual behavior. Academic Press; 2010: 253–9. https://doi.org/10.1016/B978-0-12-374387-9.00020-9.
11. Shnayder N.A., Grechkina V.V., Trefilova V.V., et al. Valproate-induced metabolic syndrome. Biomedicines. 2023; 11 (5): 1499. https://doi.org/10.3390/biomedicines11051499.
12. Shnayder N.A., Grechkina V.V., Arkhipov V.V., Nasyrova R.F. Pharmacogenetics-informed pharmacometabolomics as an innovative approach to assessing the safety and risk of pharmacotherapy with valproic acid. Safety and Risk of Pharmacotherapy. 2023; 11 (4): 450–62 (in Russ.). https://doi.org/10.30895/2312-7821-2023-386.
13. Balcı T., Çakır Biçer N., Gazeteci Tekin H., Edem P. Evaluation of the effect of parenting style and parental mealtime actions on the eating behavior of children with epilepsy. Nutrients. 2024; 16 (9): 1384. https://doi.org/10.3390/nu16091384.
14. Podlesak A.K., Mozer M.E., Smith-Simpson S., et al. Associations between parenting style and parent and toddler mealtime behaviors. Curr Dev Nutr. 2017; 1 (6): e000570. https://doi.org/10.3945/cdn.117.000570.
15. Vaughn A.E., Ward D.S., Fisher J.O., et al. Fundamental constructs in food parenting practices: a content map to guide future research. Nutr Rev. 2016; 74 (2): 98–117. https://doi.org/10.1093/nutrit/nuv061.
16. Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006; 361 (1471): 1159–85. https://doi.org/10.1098/rstb.2006.1855.
17. Shende P., Desai D. Physiological and therapeutic roles of neuropeptide Y on biological functions. Adv Exp Med Biol. 2020; 1237: 37–47. https://doi.org/10.1007/5584_2019_427.
18. Tatemoto K., Carlquist M., Mutt V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982; 296 (5858): 659–60. https://doi.org/10.1038/296659a0.
19. Allen Y.S., Adrian T.E., Allen J.M., et al. Neuropeptide Y distribution in the rat brain. Science. 1983; 221 (4613); 877–9. https://doi.org/10.1126/science.6136091.
20. Mörl K., Beck-Sickinger A.G. Intracellular trafficking of neuropeptide Y receptors. Prog Mol Biol Transl Sci. 2015; 132: 73–96. https://doi.org/10.1016/bs.pmbts.2015.02.011.
21. Bolotova N.V., Kurdiyan M.S., Filina N.Yu. Neuroendocrine mechanisms of regulation of eating behavior (review). Saratov Journal of Medical Scientific Research. 2020; 16 (3): 707–13 (in Russ.).
22. Kienast С., Gunga H.C., Steinach M. Neuropeptide Y – its role in human performance and extreme environments. Reach. 2019; 14–15: 100032. https://doi.org/10.1016/j.reach.2019.100032.
23. Zagoskin P.P., Zagoskina I.P., Savelieva N.А., Lyalyaev V.А. Modern approaches to the problem of body weight regulation. Modern Technologies in Medicine. 2014; 6 (3): 104–17 (in Russ.).
24. Chen G., Yang F., Wu T., et al. The stimulatory effect of cerebral intraventricular injection of cNPY on precocial feeding behavior in neonatal chicks (Gallus domesticus). PLoS One. 2016; 11 (4): e0153342. https://doi.org/10.1371/journal.pone.0153342.
25. Levens N., Félétou M., Galizzi J., et al. NPY effects on food intake and metabolism. In: Michel M.C. (Ed.) Neuropeptide Y and related peptides. Springer; 2004: 283–325. https://doi.org/10.1007/978-3-642-18764-3_10.
26. Rafiei N., Mitchell C.S., Tedesco C.R., et al. Chemogenetic activation of arcuate nucleus NPY and NPY/AgRP neurons increases feeding behaviour in mice. Neuropeptides. 2024; 107: 102454. https://doi.org/10.1016/j.npep.2024.102454.
27. Senthilkumaran M., Koch C., Herselman M.F., Bobrovskaya L. Role of the adrenal medulla in hypoglycaemia-associated autonomic failure – a diabetic perspective. Metabolites. 2024; 14 (2): 100. https://doi.org/10.3390/metabo14020100.
28. Kumari R., Pascalau R., Wang H., et al. Sympathetic NPY controls glucose homeostasis, cold tolerance, and cardiovascular functions in mice. bioRxiv. 2023: 2023.07.24.550381. https://doi.org/10.1101/2023.07.24.550381.
29. Schwetz T.A., Ustione A., Piston D.W. Neuropeptide Y and somatostatin inhibit insulin secretion through different mechanisms. Am J Physiol Endocrinol Metab. 2013; 304 (2): E211–21. https://doi.org/10.1152/ajpendo.00374.2012.
30. Lee N.J., Oraha J., Qi Y., et al. Altered function of arcuate leptin receptor expressing neuropeptide Y neurons depending on energy balance. Mol Metab. 2023; 76: 101790. https://doi.org/10.1016/j.molmet.2023.101790.
31. Rauchenzauner M., Haberlandt E., Scholl-Bürgi S., et al. Adiponectin and visfatin concentrations in children treated with valproic acid. Epilepsia. 2008; 49 (2): 353–7. https://doi.org/10.1111/j.1528-1167.2007.01460.x.
32. Sidhu H.S., Srinivas R., Sadhotra A. Evaluate the effects of long-term valproic acid treatment on metabolic profiles in newly diagnosed or untreated female epileptic patients: a prospective study. Seizure. 2017; 48: 15–21. https://doi.org/10.1016/j.seizure.2017.03.007.
33. Barakat S., McLean S.A., Bryant E., et al. Risk factors for eating disorders: findings from a rapid review. J Eat Disord. 2023; 11 (1): 8. https://doi.org/10.1186/s40337-022-00717-4.
34. Brennan A.M., Mantzoros C.S. Drug insight: the role of leptin in human physiology and pathophysiology – emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006; 2 (6): 318–27. https://doi.org/10.1038/ncpendmet0196.
35. Aydin K., Serdaroglu A., Okuyaz C., et al. Serum insulin, leptin, and neuropeptide Y levels in epileptic children treated with valproate. J Child Neurol. 2005; 20 (10): 848–51. https://doi.org/10.1177/08830738050200101501.
36. Rauchenzauner M., Haberlandt E., Scholl-Bürgi S., et al. Effect of valproic acid treatment on body composition, leptin and the soluble leptin receptor in epileptic children. Epilepsy Res. 2008; 80 (2–3): 142–9. https://doi.org/10.1016/j.eplepsyres.2008.03.017.
37. Kanemura H., Sano F., Maeda Y., et al. Valproate sodium enhances body weight gain in patients with childhood epilepsy: a pathogenic mechanisms and open-label clinical trial of behavior therapy. Seizure. 2012; 21 (7): 496–500. https://doi.org/10.1016/j.seizure.2012.05.001.
38. Qiao L., Schaack J., Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006; 147 (2): 865–74. https://doi.org/10.1210/en.2005-1030.
39. Elmslie J.L., Porter R.J., Joyce P.R., et al. Comparison of insulin resistance, metabolic syndrome and adiponectin in overweight bipolar patients taking sodium valproate and controls. Aust N Z J Psychiatry. 2009; 43 (1): 53–60. https://doi.org/10.1080/00048670802534341.
40. Aly R.H., Amr N.H., Saad W.E., Megahed A.A. Insulin resistance in patients on valproic acid: relation to adiponectin. Acta Neurol Scand. 2015; 131 (3): 169–75. https://doi.org/10.1111/ane.12313.
41. Abdalla M.M.I. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022; 10 (30): 10840–51. https://doi.org/10.12998/wjcc.v10.i30.10840.
42. Abdalla I.M.M. Ghrelin – physiological functions and regulation. Eur Endocrinol. 2015; 11 (2): 90–5. https://doi.org/10.17925/EE.2015.11.02.90.
43. Wang L., Su Z., Li Y.C., et al. Relationship of glucagon-like peptide 1 and peptide YY with catch-up growth in children born small for gestational age. J Clin Res Pediatr Endocrinol. 2024; 16 (1): 69–75. https://doi.org/10.4274/jcrpe.galenos.2023.2023-5-21.
44. Martin C.K., Han H., Anton S.D., et al. Effect of valproic acid on body weight, food intake, physical activity and hormones: results of a randomized controlled trial. J Psychopharmacol. 2009; 23 (7): 814–25. https://doi.org/10.1177/0269881108091595.
45. Salehi M., Purnell J.Q. The role of glucagon-like peptide-1 in energy homeostasis. Metab Syndr Relat Disord. 2019; 17 (4): 183–91. https://doi.org/10.1089/met.2018.0088.
46. Shnayder N.A., Grechkina V.V., Khasanova A.K., et al. Therapeutic and toxic effects of valproic acid metabolites. Metabolites. 2023; 13 (1): 134. https://doi.org/10.3390/metabo13010134.
47. Lagrange A.H. Valproate enhances neuropeptide y expression: modulating the modulators. Epilepsy Curr. 2007; 7 (4): 107–9. https://doi.org/10.1111/j.1535-7511.2007.00167.x.
48. Torlasco M.B., Estrin M.A. Valproic acid and weight gain in patients receiving antiepileptic treatment: a systematic review. Rev Inform Cientif. 2023; 102: 1–16 (in Spanish). https://doi.org/10.5281/zenodo.7843532.
49. Cansu A., Serdaroglu A., Camurdan O., et al. Serum insulin, cortisol, leptin, neuropeptide Y, galanin and ghrelin levels in epileptic children receiving valproate. Horm Res Paediatr. 2011; 76 (1): 65–71. https://doi.org/10.1159/000327367.
50. Tokgoz H., Aydin K., Oran B., Kiyici A. Plasma leptin, neuropeptide Y, ghrelin, and adiponectin levels and carotid artery intima media thickness in epileptic children treated with valproate. Childs Nerv Syst. 2012; 28 (7): 1049–53. https://doi.org/10.1007/s00381-012-1788-7.
51. Çiçek N.P., Kamaşak T., Serin M., et al. The effects of valproate and topiramate use on serum insulin, leptin, neuropeptide Y and ghrelin levels in epileptic children. Seizure. 2018; 58: 90–5. https://doi.org/10.1016/j.seizure.2018.03.013.
52. Berkman N.D., Brownley K.A., Peat C.M., et al. Management and outcomes of binge-eating disorder. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. Available at: https://www.ncbi.nlm.nih.gov/books/NBK338301/ (accessed 15.07.2024).
53. The hunger-satiety scale. Available at: https://uhs.berkeley.edu/sites/default/files/wellness-hungersatietyscale.pdf (accessed 15.07.2024).
54. Paravidino V.B., Mediano M.F.F., Silva I.C.M., et al. Effect of physical exercise on spontaneous physical activity energy expenditure and energy intake in overweight adults (the EFECT study): a study protocol for a randomized controlled trial. Trials. 2018; 19 (1): 167. https://doi.org/10.1186/s13063-018-2445-6.
55. Experts’ consensus on the interdisciplinary approach towards the management, diagnostics, and treatment of patients with metabolic syndrome. Cardiovascular Therapy and Prevention. 2013; 12 (6): 41–81.
56. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285 (19): 2486–97. https://doi.org/10.1001/jama.285.19.2486.
57. Grundy S.M., Cleeman J.I., Daniels S.R., et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112 (17): 2735–52. https://doi.org/10.1161/CIRCULATIONAHA.105.169404.
58. Neznanov N.G. A paradigm shift to treat psychoneurological disorders. Personalized Psychiatry and Neurology. 2021; 1 (1): 1–2.
About the Authors
N. A. ShnayderRussian Federation
Natalia A. Shnayder - Dr. Sci. Med., Prof.
3 Bekhterev Str., Saint Petersburg, 192019; 1 Partizan Zheleznyak Str., Krasnoyarsk, 660022
WoS ResearcherID M-7084-2014, Scopus Author ID 24503222300
V. V. Grechkina
Russian Federation
Violetta V. Grechkina.
3 Bekhterev Str., Saint Petersburg, 192019; 9I Obvodnoy Canal Emb., Saint Petersburg, 191167
Scopus Author ID 58076147700
M. Ya. Kissin
Russian Federation
Mikhail Yа. Kissin - Dr. Sci. Med., Prof.
9I Obvodnoy Canal Emb., Saint Petersburg, 191167; 6-8 Lev Tolstoy Str., Saint Petersburg, 197022
D. V. Dmitrenko
Russian Federation
Diana V. Dmitrenko - Dr. Sci. Med.
1 Partizan Zheleznyak Str., Krasnoyarsk, 660022
WoS ResearcherID H-7787-2016
R. F. Nasyrova
Russian Federation
Regina F. Nasyrova - Dr. Sci. Med.
3 Bekhterev Str., Saint Petersburg, 192019; 92 Lenin Ave., Tula, 300012
WoS ResearcherID H-7787-2016
Review
For citations:
Shnayder N.A., Grechkina V.V., Kissin M.Ya., Dmitrenko D.V., Nasyrova R.F. Role of neuropeptide Y in development of valproate-induced eating behaviour disorder. Epilepsy and paroxysmal conditions. 2024;16(4):349-361. https://doi.org/10.17749/2077-8333/epi.par.con.2024.207
JATS XML

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.







































