Scroll to:
Anticonvulsants impacting bone metabolism: interim results from a cross-sectional study
https://doi.org/10.17749/2077-8333/epi.par.con.2024.202
Abstract
Background. Despite a wide range of antiepileptic drugs (AEDs) with an improved pharmacological profile, patients often experience a variety of side effects during long-trem anticonvulsant therapy, among which are osteoporotic disorders. Currently, the mechanisms of AED effect on bone metabolism remain poorly understood, which creates certain difficulties in prevention and treatment of AED-induced osteoporosis.
Objective: to study bone mineral density and laboratory parameters of bone metabolism in patients with epilepsy and longterm AED administration.
Material and methods. A cross-sectional study included two comparison groups: 100 adult patients with epilepsy receiving AEDs for more than 12 months and 58 healthy volunteers without taking AEDs. All participants underwent general clinical examination, computed tomography (CT) densitometry at three time points (L1, L2 and femoral neck) and laboratory tests of mineral metabolism.
Results. According to CT-densitometry results, a decrease in bone mineral density was detected in the majority of participants from both study groups. While assessing an impact of osteoporosis risk factors on bone tissue in epileptic patients, low motor activity and duration of AED therapy were the most significant, which was associated with lower bone mineral density indices. The study of laboratory mineral metabolism indicators revealed significant inter-group differences in indicators such as ionized calcium, 25-hydroxy-calciferol, free thyroxine and prolactin (p(U)=0.044, p(U)=0.040, p(U)=0.001, p(U)=0.003, respectively).
Conclusion. The intermediate study results showed that long-term anticonvulsant use negatively affected bone metabolism in patients suffering from epilepsy. The data obtained point at need for further in-depth study of AED therapy effect on mineral metabolism.
Keywords
For citations:
Sivakova N.A., Abramova I.V., Trukhina I.Yu., Rybasova V.P., Kasyanov E.D., Lukina L.V., Mikhailov V.A., Mazo G.E. Anticonvulsants impacting bone metabolism: interim results from a cross-sectional study. Epilepsy and paroxysmal conditions. 2024;16(3):192–201. https://doi.org/10.17749/2077-8333/epi.par.con.2024.202
INTRODUCTION / ВВЕДЕНИЕ
Over the past decades, the landmark advances have been made in epilepsy diagnostics and treatment, but patients are forced to take antiepileptic drugs (AEDs) long-term or lifelong. Despite a wide range of anticonvulsants with improved pharmacological profile and high safety, patients often experience a variety of adverse effects during treatment. Recent studies suggest that a negative effect of AED therapy may be manifested by pathological osteoresorption, when bones lose density and are prone to fractures [1][2].
The prevalence of pathological fractures among epilepsy patients is 2–6 times higher than in general population, which risk elevates with increasing duration of anticonvulsant therapy [3]. At present, the mechanisms of AED effect on bone metabolism remain poorly studied that creates certain difficulties in prevention and treatment of anticonvulsant-induced osteoporosis. A decline in bone mineral density (BMD), development of osteoporotic disorders and, consequently, frequent fractures in epilepsy patients is a pressing and understudied issue.
Within the framework of the research project “An impact of anticonvulsants on developing osteoporosis in epilepsy patients”, conducted at Bekhterev National Medical Research Center for Psychiatry and Neurology (Bekhterev NMRC PN), the pilot data were obtained while assessing a cohort of 37 patients with epilepsy. The data showed that long-term anticonvulsant use tended to reduce BMD level and that multiple exogenous and endogenous cues such as such as alcohol, smoking, previous fractures, physical activity, gender, age might influence mineral metabolism [4][5]. Altogether, it corroborates a need to comprehensively assess mineral metabolism and BMD in patients with epilepsy as well as comparatively analyze with those in control group (somatically healthy participants not taking anticonvulsants).
Late diagnostics of osteoporosis results in deteriorated quality of patients’ life, unfavorable prognosis, increased disability rate and medical costs. Studies aimed at studying AED effect on BMD and bone metabolism are of great importance because they allow to gain deeper insights into mechanisms underlying development of patient osteoporotic disorders and design monitoring and treatment strategies to minimize their impact.
Objective: to assess BMD and laboratory bone metabolism parameters in epilepsy patients after long-term AED administration.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
A cross-sectional study with two comparison groups was conducted: main group of adult epilepsy patients (EPs) receiving AEDs for more than 12 months, and control group of apparently healthy participants (HPs) not receiving AEDs. Patients with epilepsy underwent inpatient and outpatient treatment at the Department of Treatment of Exogenous Organic Disorders and Epilepsy at Bekhterev National Medical Research Center for Psychiatry and Neurology.
Inclusion, non-inclusion and exclusion criteria / Критерии включения, невключения и исключения
Inclusion criteria
Main group:
– aged 21 to 60 years inclusive;
– verified epilepsy diagnosis;
– at least 12 month-long epilepsy and AED administration;
– ability to read, understand and sign informed consent to participate in the study.
Control group:
– aged 21 to 60 years inclusive;
– no AED administration;
– ability to read, understand and sign informed consent to participate in the study.
Non-inclusion criteria
For all study groups:
– aged 21 to 60 years;
– patient or legal representative / healthy subject refusal to participate in the study;
– positive pregnancy test;
– clinically significant decompensated somatic or mental diseases;
– drug administration affecting BMD (glucocorticosteroids, antidepressants, thyroid hormones, lithium preparations, neuroleptics, high heparin dose preparations, proton pump inhibitors, etc.);
– severe cognitive impairment manifested by participant's inability to read and understand informed consent to participate in the study.
Exclusion criteria
For all study groups:
– refusal to comply with study protocol, or patient withdrawal of consent;
– positive pregnancy test;
– onset of treatment by antidepressants, antipsychotics, corticosteroids, heparin preparations, hormone replacement therapy for medical reasons;
– decompensated somatic and/or mental diseases interfering with participation in the study.
General characteristics of study groups / Общая характеристика исследуемых групп
Main group (EPs) consisted of 100 adult patients (median age 36 (29–43) years), including 53 (53%) females and 47 (47%) males; verified epilepsy in all patients with an average disease duration of 10 (4–17) years. Control group (HPs) included 58 subjects (median age 29 (25–43) years), including 42 (72%) females and 16 (27.6%) males.
Age and gender participant distribution shows that main group (EPs) is characterized by an older mean age and a more equal female/male ratio compared to control group (HPs), with higher female/male ratio and lower median age. Such differences may be important while analyzing and interpreting study data.
AED-generation allowed to stratify patients from main group into two subgroups: AC1 – traditional anticonvulsants (carbamazepine, valproic acid, benzobarbital, oxcarbazepine, phenobarbital) and AC2 – next-latest generation anticonvulsants (levetiracetam, lacosamide, lamotrigine). Subgroup AC1 consisted of 40 patients including 21 (52.5%) males and 19 (47.5%) females, median age 36 (32–39) years. Subgroup AC2 consisted of 60 subjects including 26 (43.3%) males and 34 (56.7%) females, median age 37 (34–42) years.
Examination methods / Методы обследования
All participants underwent a clinical examination, including a detailed medical history collection and assessment of osteoporosis risk factors, laboratory testing for trace elements and bone metabolism hormones as well as BMD radiological examination by using quantitative computed tomography (CT densitometry).
To assess BMD, X-ray computer osteodensitometry was performed using a multidetector computed tomograph Aquilion One 640 (Canon, Japan) at three points: lumbar vertebrae L1, L2 and femoral neck. The data analysis was performed using densitometric T- and Z-criteria.
In laboratory tests, the following mineral metabolism parameters were analyzed: thyroid-stimulating hormone, total triiodothyronine, free triiodothyronine, total thyroxine, free thyroxine, prolactin (enzyme-linked immunosorbent assay (ELISA), Vector-best-Baltika (Russia)), parathyroid hormone, 25-hydroxycalciferol (ELISA, DRG International (USA)), N-osteocalcin (ELISA; IImmunodiagnostic Systems (UK)), alkaline phosphatase, total calcium, phosphorus (spectrophotometry; Randox Laboratories Ltd (UK)), ionized calcium (ion-selective potentiometry; Roche (Switzerland)).
All participants were assessed for physical activity (PA) level using the Physical Activity Questionnaire (PAQ 23+) [6].
Statistical analysis / Статистический анализ
Data statistical analysis was performed using Stattech software version 3.1.10 (Stattech LLC, Russia). Quantitative indicators were assessed for normal distribution by using Shapiro–Wilk test. Quantitative indicators with normal distribution were presented as arithmetic mean (M) and standard deviation (SD), 95% confidence interval (95% CI) boundaries. In case of non-normal distribution, quantitative data were presented as median (Me) together with the lower and upper quartiles (Q25; Q75). Categorical data were presented as absolute value and percentage. Student's t-test and Mann–Whitney U-test as well as Kruskal–Wallis and Pearson's χ2 tests were used for group comparison. Correlation was assessed by using Spearman's rank correlation coefficient and Chaddock scale. Linear regression was applied to analyze a diagnostic significance of quantitative traits for specific outcome prediction. Differences were considered significant at p<0.05 level.
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
An impact of osteoporosis risk factors on bone mineral density / Влияние риск-факторов остеопороза на МПКТ
Smoking and alcohol consumption
In long-term AED administration group, 35 (35%) patients regularly used tobacco, including electronic cigarettes and tobacco heating systems, whereas in control group – 16 (27.6%) participants. Comparative analysis of tobacco smoking effect on BMD revealed no significant changes in CT bone density indices in smoking and nonsmoking participants between main and control groups (p(χ2)=0.554 and p(χ2)=0.141, respectively) (Table 1).
Table 1. Comparatively analyzed effect of osteoporosis risk factors (smoking, alcohol consumption, motor activity) on bone mineral density (BMD) changes in epilepsy patients (EPs) and healthy participants (HPs)
Таблица 1. Сравнительный анализ влияния риск-факторов остеопороза (табакокурение, употребление алкоголя, двигательная активность) на изменения минеральной плотности костной ткани (МПКТ) в группах больных эпилепсией (БЭ) и здоровых добровольцев (ЗД)
Group / Группа | Category / Категория | BMD, n (%) / МПКТ, n (%) | p (χ2) | ||
Normal range /Норма | Osteopenia /Остеопения | Osteoporosis /Остеопороз | |||
Smoking / Табакокурение | |||||
EPs / БЭ | Yes / Курит | 21 (60,0) | 9 (25,7) | 5 (14,3) | 0,554 |
No / Не курит | 32 (49,2) | 23 (35,4) | 10 (15,4) | ||
HPs / ЗД | Yes / Курит | 5 (31,2) | 7 (43,8) | 4 (25,0) | 0,141 |
No / Не курит | 24 (57,1) | 14 (33,3) | 4 (9,5) | ||
Alcohol consumption / Употребление алкоголя | |||||
EPs / БЭ | No / Не употребляет | 36 (67,9) | 26 (81,2) | 12 (80) | 0,406 |
<3 times a week / <3 раз в неделю | 6 (11,3) | 1 (3,1) | 0 (0,0) | ||
≥3 times a week / ≥3 раз в неделю | 11 (20,8) | 5 (15,6) | 3 (20,0) | ||
HPs / ЗД | No / Не употребляет | 24 (82,0) | 9 (42,9) | 1 (12,5) | 0,003* |
<3 times a week / <3 раз в неделю | 1 (3,4) | 3 (14,3) | 2 (25,0) | ||
≥3 times a week / ≥3 раз в неделю | 4 (13,8) | 9 (42,9) | 5 (62,5) | ||
Motor activity level / Уровень двигательной активности | |||||
EPs / БЭ | Very high / Очень высокая | 3 (100,0) | 0 (0,0) | 0 (0,0) | <0,001** |
High / Высокая | 28 (77,8) | 5 (13,9) | 3 (8,3) | ||
Moderate / Умеренная | 20 (47,6) | 18 (42,9) | 4 (9,5) | ||
Low / Низкая | 1 (12,5) | 4 (50,0) | 3 (37,5) | ||
Very low / Очень низкая | 1 (9,1) | 5 (45,5) | 5 (45,5) | ||
HPs / ЗД | Very high / Очень высокая | 2 (100,0) | 0 (0,0) | 0 (0,0) | 0,120 |
High / Высокая | 19 (54,3) | 13 (37,1) | 3 (8,6) | ||
Moderate / Умеренная | 8 (40,0) | 8 (40,0) | 4 (20,0) | ||
Low / Низкая | 0 (0,0) | 0 (0,0) | 1 (100,0) | ||
Very low / Очень низкая | 0 (0,0) | 0 (0,0) | 0 (0,0) | ||
Note. Significant differences (p<0.05) are highlighted in bold. * p(χ2)=0.026 while comparing normal and osteopenia subgroups; p(χ2)=0.002 while comparing normal and osteoporosis subgroups. ** p(χ2)=0.017 while comparing high and low physical activity subgroups; p(χ2)=0.002 while comparing high and very low physical activity subgroups.
Примечание. Полужирным шрифтом выделены статистически значимые различия (p<0,05). * p(χ2)=0,026 при сравнении подгрупп нормы и остеопении; p(χ2)=0,002 при сравнении подгрупп нормы и остеопороза. ** p(χ2)=0,017 при сравнении подгрупп высокой и низкой двигательной активности; p(χ2)=0,002 при сравнении подгрупп высокой и очень низкой двигательной активности.
To assess an effect of alcohol consumption on CT densitometry parameters, subgroups were stratified based on degree of BMD CT changes, with analyzing frequency of alcohol consumption in each subgroup. It should be noted that patients receiving AED therapy were aware that the underlying disease and anticonvulsant therapy imply complete abstinence from alcohol, however, in each subgroup there were respondents who admitted to drinking alcohol.
In normal BMD subgroup, 36 (67.9%) participants had never alcohol consumption, 6 (11.3%) subjects drink alcohol up to 3 times a week, and 11 (20.8%) subjects – more than 3 times a week. In osteoporosis BMD subgroup, 26 (81.2%) patients do not drink alcohol, 1 (3.1%) subject drinks alcohol up to 3 times a week, and 5 (15.6%) – drink more than 3 times a week. In CT-based osteoporosis group, 12 (80%) subjects do not drink alcohol, and 3 (20%) drink alcohol more than 3 times a week. According to the data obtained, no significant effect of frequency of alcohol consumption on BMD changes was found in main group (p(χ2)=0.406). Assessing an effect of alcohol consumption on BMD in control group also showed no significant decline in CT bone density indices due to increased frequency of alcohol consumption (p(χ2)=0.003) (see Table 1).
The data obtained are not in line with those reported by A. Vergatti et al. showing that a non-smoker subgroup had lower prevalence of radiographic osteoporosis and hypovitaminosis D signs compared to active and passive smokers (p<0.05) [7]. Also, a meta-analysis conducted by Z. Cheraghi et al. found that individuals who consume 0.5–1 drink per day had the risk of developing osteoporosis by 1.38-fold higher compared to those who do not consume alcohol [8]. Nonetheless, the study by D.R. Baddoo et al. with 835 epileptic patients and long-term anticonvulsant use revealed no significant effect of smoking and alcohol on BMD: p(χ2)=0.83, p(χ2)=0.76, respectively [9]. The contradictory results obtained in various studies point at a to conduct further detailed investigation assessing an effect of smoking and alcohol consumption on BMD level related to long-term AED therapy as well as in healthy volunteers.
Physical activity
PA assessment using PAQ 23+ found out that patients with low and very low PA levels have a more significant decline in BMD compared to those who adhere to a more active lifestyle. Of the 11 patients with very low PA, 10 (91%) had BMD changed to osteopenia and osteoporosis level. Among 36 patients with high PA levels, only 8 subjects (22%) had lowered BMD level. Significant differences were found (high vs. low PA subgroups, p(χ2)<0.001, p=0.017; high vs. very low PA subgroups, p=0.002), suggesting about a significant relation between PA level and BMD change magnitude (see Table 1).
The data obtained are probably related to the prevalence of high PA in control group comprising 66 (54–78) points corresponding to high PAQ 23+ score. According to the 2016 report of the International League Against Epilepsy (ILAE) Task Force on Sports and Epilepsy, patients with seizures are often discouraged from engaging in active sports due to fear, overprotection, or lack of awareness [10]. However, physical activity and sports contribute to improved patients’ general condition including higher self-esteem, socialization and improved health long term [10, 11]. A study assessing BMD in 500 women over 40 years of age also showed high odds of normal bone density with moderate (p=0.053) and high (p<0.001) PA level [12]. Hence, patients with PA level below moderate are more susceptible to decreased BMD than patients with high PA level seemingly because mechanical effects such as of muscle contractions and body gravity on bones and joint tissues lead to activation of osteocytes producing signaling molecules, which stimulate osteoblast differentiation [13, 14].
CT densitometry / КТ-денситометрическое обследование
General group-specific BMD characteristics
BMD level assessed by CT densitometry showed that it was decreased in 47 (47%) patients including 32 (32%) participants with CT osteopenia signs, 15 (15%) – CT osteoporosis signs. In control group, BMD changes were detected in 29 participants (50%), of which CT osteopenia and osteoporosis signs were noted in 21 (36.2%) and 8 (13.8%) subjects, respectively.
A comparatively analyzed frequency of BMD CT changes in both groups found no significant differences (p(χ2)=0.863). The study by V. Chandrasekaran et al. involved 926 males and 1070 females, of whom 1.8% and 1.9%, respectively, used anticonvulsants and showed lower BMD after AED administration [2]. In our work, no significant inter-group differences in BMD indices were found, which potentially requires to conduct larger-scale study with risk factor adjustment.
Duration of AED administration and impact on BMD level
BMD level change was assessed in connection with duration of AED administration in main group. It was shown that patients with normal BMD level, average anticonvulsant use was 4 years, whereas those with BMD decreased to osteopenia and osteoporosis level – 15 and 10 years, respectively (Fig. 1). BMD significantly decreased to osteopenia and osteoporosis level was found in patients during longer-term AEDs therapy (p(H)<0.001).
A correlation analysis was performed to assess a relation between decreased BMD level and AED therapy duration. According to the Chaddock scale, a significant inverse correlation (ρ=–0.626, p<0.001) was found between decreased densitometric index “L1/L2 Z-score” and anticonvulsant therapy duration (Fig. 2). Moreover, it also showed that AED therapy duration increased by 1 point corresponds to the expected decline in the “Z-score” by 0.196. The data obtained evidence about high risk of BMD decline with longer anticonvulsant therapy.
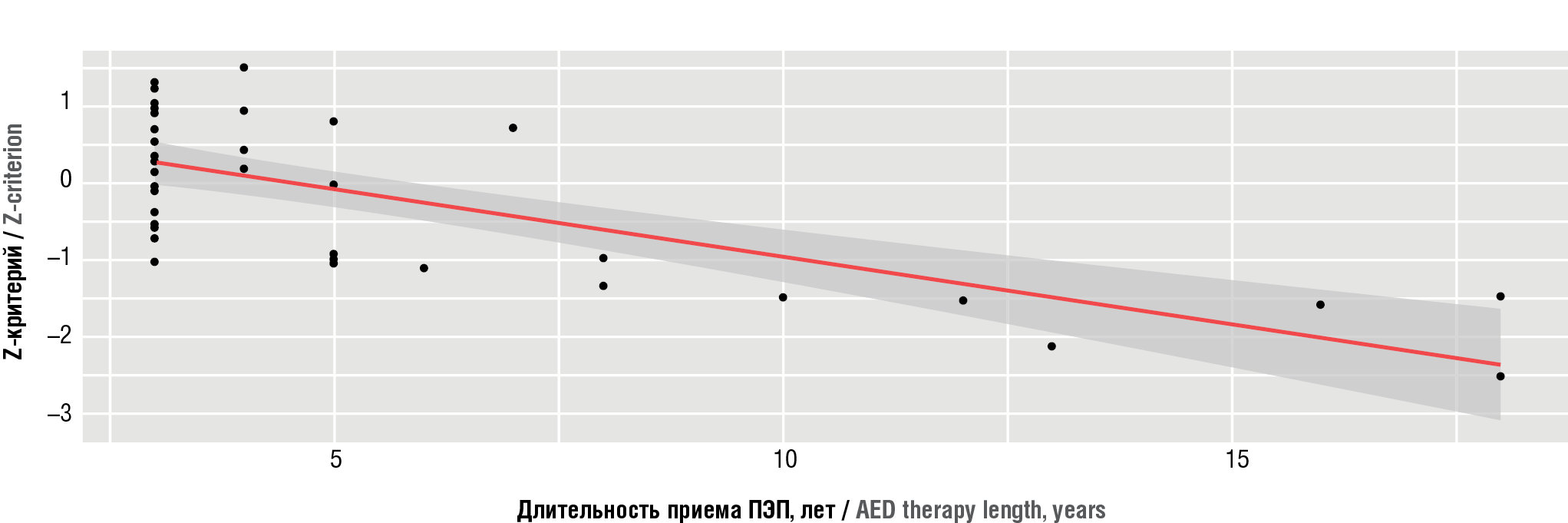
Figure 1. Analysed bone mineral density (BMD) changes depending on antiepileptic drug (AED) therapy length
Рисунок 1. Анализ изменений минеральной плотности костной ткани (МПКТ) в зависимости от длительности приема противоэпилептических препаратов (ПЭП)
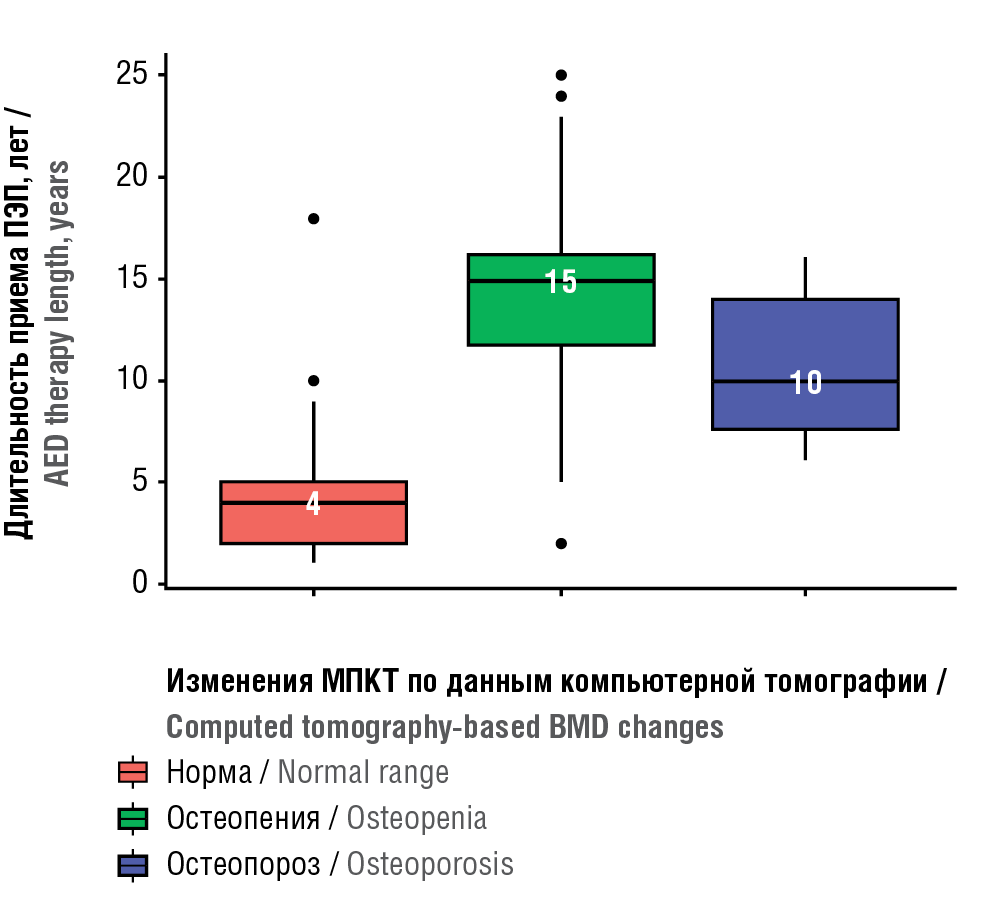
Figure 2. Regression graph showing a relation between bone mineral density (Z-criterion) on the duration of antiepileptic drug (AED) administration in epileptic patients
Рисунок 2. График регрессии, показывающий зависимость показателя «минеральная плотность костной ткани» (Z-критерий) от длительности приема противоэпилептических препаратов (ПЭП) в группе больных эпилепсией
AED generation influence on BMD level
CT densitometry data showed that in AC1 subgroup, 6 (15%) and 15 (37%) patients had BMD lowered to osteoporosis and osteopenia level, respectively whereas in AC2 subgroup, 8 (13.6%) subjects – to osteoporosis and osteopenia level, respectively. While analyzing a relation between BMD decrease level and AED generation use, no significant differences were found (p(χ2)=0.863). The data obtained may evidence about a negative effect of anticonvulsants of different generations on bone metabolism. Perhaps, additional larger-scale studies and a long-term follow-up period may allow a better understanding of how AED therapy affects bone condition.
Laboratory examination / Лабораторное исследование
A comparative analysis of laboratory parameters affecting mineral metabolism was conducted in both study groups.
When comparing the average ionized calcium, free thyroxine and prolactin level, significant differences were found between main and control group (p(U)=0.044, p(U)=0.001, p(U)=0.003, respectively), which may point at dominance osteoresorption over osteogenesis in main group. Significant inter-group differences in vitamin D levels (p(U)=0.040) were also found.
The study data agree with numerous studies showing that induction of liver cytochrome P450 system (CYP450) accelerates vitamin D catabolism into its polar inactive metabolites as well as weakens its biologically active counterparts [15, 16]. It accounts for anticonvulsant effect as liver cytochrome P450 inducers on bone metabolism. However, the mechanisms of action of the latter and AED impact are understudied [16].
Other laboratory parameters showed no significant inter-group differences (Table 2).
Table 2. Comparison of “micronutrients” and “hormones” laboratory parameters in epilepsy patients (EPs) and healthy participants (HPs)
Таблица 2. Сравнение лабораторных показателей «микроэлементы» и «гормоны» в группах здоровых добровольцев (ЗД) и больных эпилепсией (БЭ)
Indicator / Показатель | Group / Группа | n | Me [Q25; Q75] | p* |
Ionized calcium, mmol/l // Ионизированный | EPs / БЭ | 100 | 1,21 [ 1,19; 1,25] | 0,044 |
HPs /ЗД | 58 | 1,23 [ 1,21; 1,26] | ||
Vitamin D, ng/ml // Витамин D, нг/мл | EPs / БЭ | 100 | 20,229 [ 10,395; 28,495] | 0,040 |
HPs /ЗД | 58 | 21,933 [ 16,826; 29,375] | ||
Total calcium, mmol/l // Общий кальций, ммоль/л | EPs / БЭ | 100 | 2,48 [ 2,36; 2,58] | 0,612 |
HPs /ЗД | 58 | 2,49 [ 2,42; 2,56] | ||
Phosphorus, mmol/l // Фосфор, ммоль/л | EPs / БЭ | 100 | 1,07 [ 0,98; 1,19] | 0,287 |
HPs /ЗД | 58 | 1,02 [ 0,93; 1,16] | ||
Free triiodothyronine, nmol/l // Трийодтиронин свободный, нмоль/л | EPs / БЭ | 100 | 5,508 [ 5,002; 6,506] | 0,853 |
HPs /ЗД | 58 | 5,913 [ 5,141; 6,285] | ||
Total triiodothyronine, pmol/l // Трийодтиронин | EPs / БЭ | 100 | 1,656 [ 1,279; 1,869] | 0,078 |
HPs /ЗД | 58 | 1,731 [ 1,582; 1,899] | ||
Total thyroxine, pmol/l // Тироксин общий, пмоль/л | EPs / БЭ | 100 | 86,500 [ 69,609; 113,181] | 0,665 |
HPs /ЗД | 58 | 87,491 [ 78,704; 98,260] | ||
Free thyroxine, nmol/l // Тироксин свободный, нмоль/л | EPs / БЭ | 100 | 15,331 [ 14,114; 17,028] | 0,001 |
HPs /ЗД | 58 | 16,564 [ 15,514; 18,127] | ||
Thyroid stimulating hormone, mIU/l // | EPs / БЭ | 100 | 1,866 [ 1,268; 2,851] | 0,903 |
HPs /ЗД | 58 | 1,841 [ 1,217; 3,125] | ||
Alkaline phosphatase, U/l // Щелочная | EPs / БЭ | 100 | 174 [ 139; 226] | 0,828 |
HPs /ЗД | 58 | 169 [ 144; 226] | ||
Parathyroid hormone, pmol/l // Паратиреоидный гормон, пмоль/л | EPs / БЭ | 100 | 49,178 [ 39,306; 65,130] | 0,385 |
HPs /ЗД | 58 | 50,763 [ 41,861; 67,659] | ||
N-osteocalcin, ng/ml // N-остеокальцин, нг/мл | EPs / БЭ | 100 | 11,179 [ 8,573; 16,937] | 0,967 |
HPs /ЗД | 58 | 11,596 [ 9,303; 15,274] | ||
Prolactin, mIU/ml // Пролактин, мМЕ/мл | EPs / БЭ | 100 | 454 [ 273; 596] | 0,003 |
HPs /ЗД | 58 | 278 [ 179; 460] |
Note. * Mann–Whitney U test. Significant differences (p<0.05) are highlighted in bold.
Примечание. * U–критерий Манна–Уитни. Полужирным шрифтом выделены статистически значимые различия (p<0,05).
In main and control group, median prolactin level was 278 (179–460) mg/ml and 454 (273–596) mg/ml, respectively. Increased blood prolactin in patients with epilepsy may be related to both epileptic seizures per se and anticonvulsant use [11][17][18]. The mechanism by which epileptic seizures contribute to hyperprolactinemia is not fully understood. However, it is believed that it is associated with hypothalamic-pituitary-adrenal axis activation subsequently leading to increased blood prolactin levels [11, 18]. The results of rat study by Y. Panahi et al. show that after 10 week-long chronic seizures, higher blood serum prolactin level is observed [19]. M.F. Wang investigated 110 patients with epilepsy and found that post- vs. pre-seizure prolactin level in the blood serum was significantly increased and reached a level more than 5 times exceeding the baseline level in 59 patients [20].
The lowered blood free thyroxine level after long-term AED administration we showed here is in line with meta-analysis data reported by Y.X. Zhang et al. analyzing 35 studies and a total of 997 patients. This analysis showed that the use of traditional anticonvulsants (carbamazepine and valproic acid) results in significantly decreased serum levels of triiodothyronine, thyroxine and increase in thyroid-stimulating hormone level [21].
While assessing laboratory parameters affecting bone metabolism in connection with AED generation, significant difference in prolactin levels was found. The average prolactin concentration of significantly higher among patients taking traditional anticonvulsants (AC1 – 478 (282–630) mg/ml, AC2 – 445 (266–544) mg/ml, p(H)=0.01)). In the work by W.S. Mohamed et al. with 50 epilepsy males, prolactin level was also significantly increased in group of patients taking valproic acid vs. levetiracetam [22]. Valproate-induced hyperprolactinemia results from altered regulation of GABA1-ergic, noradrenergic, and serotonergic neurons, which in turn modulate dopamine release [23].
The data obtained may shed light on a trajectory of further studies analyzing an effect of different AED generations on blood prolactin level in patients with epilepsy and impaired BMD. According to the meta-analysis reported by Y.X. Zhang et al., a significantly decreased blood free thyroxine level was found in patients taking carbamazepine, phenytoin and valproic acid included in group of traditional anticonvulsants compared with group of patients taking next-generation AEDs [21]. In the work by F.Y. Shih et al. assessing 298 patients with epilepsy and no former thyroid pathologies, it was concluded that carbamazepine use is an independent risk factor for decreased free thyroxine level in the blood [24]. In our study, thyroxine level was also significantly reduced in patients taking both traditional and new-generation AEDs compared to control group (p(H)=0.018 while comparing control vs. old-generation AED subgroup, main group; p(H)=0.018 upon comparison between control group and new-generation AED subgroup, main group.
The laboratory data confirm that one of the mechanisms whereby anticonvulsants may target bone metabolism relies on their impact on hormonal metabolism. Moreover, such observations may evidence about distinct mechanisms related to AED of different generations on on mineral metabolism pointing at a need for conducting a more comprehensive study. Obviously, a correction of BMD indices upon administration of AEDs of different generations should be based on their molecular impact.
Research perspective / Перспектива исседования
Here, we present interim data obtained within the research project “An impact of anticonvulsants on developing osteoporosis in epilepsy patients” 2022–2024). The recruitment of study participants including gender- and age-matched groups 200 epilepsy patients and 100 healthy volunteers is currently being completed.
Resulting from a comprehensive study of patients with epilepsy during long-term anticonvulsant administration, there will be obtained data reflecting a skeletal system state in study participants as well as radiological, endocrinological and metabolic parameters, lifestyle preferences associated with osteoporosis risk. Obtaining endpoint data may allow to create a model for assessing osteoporosis risks in patients during long-term AED therapy.
CONCLUSION / ЗАКЛЮЧЕНИЕ
The interim study results showed that long-term anticonvulsant use negatively affects mineral metabolism in patients with epilepsy. Thus, our work underlines an importance of BMD and bone metabolism marker monitoring during AED administration as well as a need to develop individualized approaches to prevent and treat anticonvulsant-induced osteoporosis. In addition, in-depth multicenter longitudinal studies are required to reveal more precise mechanisms of AED action on bone tissue.
1 GABA – gamma aminobutyric acid.
References
1. Shen C., Chen F., Zhang Y., et al. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone. 2014; 64: 246–53. https://doi.org/10.1016/j.bone.2014.04.018.
2. Chandrasekaran V., Pasco J.A., Stuart A.L., et al. Anticonvulsant use and bone health in a population-based study of men and women: cross-sectional data from the Geelong Osteoporosis Study. BMC Musculoskelet Disord. 2021; 22 (1): 172. https://doi.org/10.1186/s12891-021-04042-w.
3. Ecevit C., Aydoğan A., Kavakli T., Altinöz S. Effect of carbamazepine and valproate on bone mineral density. Pediatr Neurol. 2004; 31 (4): 279–82. https://doi.org/10.1016/j.pediatrneurol.2004.03.021.
4. Sivakova N.A., Abramova I.V., Rybasova V.P., et al. Assessment of bone mineral density in epileptic patients with long-term antiepileptic therapy: pilot data. V.M. Bekhterev Review of Psychiatry and Medical Psychology. 2023; 57 (4): 75–89 (in Russ.). https://doi.org/10.31363/2313-7053-2023-859.]
5. Sivakova N.A., Abramova I.V., Rybasova V.P., et al. Assessment of bone mineral density in epileptic patients with long-term antiepileptic therapy. All-Russian Scientific and Practical Conference with International Participation “Days of Osteoporosis in Saint Petersburg”, March 16–17, 2023, St. Petersburg. Abstract book. Osteoporosis and Bone Diseases. 2023; 26 (1S): S46–7 (in Russ.). https://doi.org/10.14341/osteo2023261S.]
6. Bubnova M.G., Aronov D.M. Methodic recommendations. Maintaining physical activity of those with limitations in health. CardioSomatics. 2016; 7 (1): 5–50 (in Russ.).]
7. Vergatti A., Abate V., D’Elia L, et al. Smoking habits and osteoporosis in community-dwelling men subjected to dual-X-ray absorptiometry: a cross-sectional study. J Endocrinol Invest. 2024 May 28. https://doi.org/10.1007/s40618-024-02402-6.
8. Cheraghi Z., Doosti-Irani A., Almasi-Hashiani A., et al. The effect of alcohol on osteoporosis: a systematic review and meta-analysis. Drug Alcohol Depend. 2019; 197: 197–202. https://doi.org/10.1016/j.drugalcdep.2019.01.025.
9. Baddoo D.R., Mills A.A., Kullab R.B., et al. Metabolic bone disease in patients with epilepsy and the use of antiepileptic drugs – insight from a Danish cross-sectional study. Seizure. 2021; 86: 29–34. https://doi.org/10.1016/j.seizure.2021.01.008.
10. Capovilla G., Kaufman K.R., Perucca E., et al. Epilepsy, seizures, physical exercise, and sports: a report from the ILAE Task Force on Sports and Epilepsy. Epilepsia. 2016; 57 (1): 6–12. http://doi.org/10.1111/epi.13261.
11. Petrov K.V., Petrova M.M., Shnayder N.A., Nasyrova R.F. Mechanisms of action and safety of exercise in patients with epilepsy (review). Bulletin of Rehabilitation Medicine. 2021; 6: 81–91 (in Russ.). https://doi.org/10.38025/2078-1962-2020-100-6-81-91.]
12. Kopiczko A. Determinants of bone health in adults Polish women: the influence of physical activity, nutrition, sun exposure and biological factors. PLoS One. 2020; 15 (9): e0238127. https://doi.org/10.1371/journal.pone.0238127.
13. Santos A., Bakker A.D., Zandieh-Doulabi B., et al. Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res. 2009; 27 (10): 12807. https://doi.org/10.1002/jor.20888.
14. Robling A.G., Turner C.H. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009; 19 (4): 319–38. https://doi.org/10.1615/critreveukargeneexpr.v19.i4.50.
15. Dontseva E.A., Pilipenko P.I., Shnayder N.A., et al. Prevalence of anticonvulsant-induced vitamin D deficiency. Epilepsia i paroksizmal'nye sostoania / Epilepsy and Paroxysmal Conditions. 2022; 14 (3): 304–15 (in Russ.). https://doi.org/10.17749/2077-8333/ epi.par.con.2022.117.]
16. Zhidkova I.A., Kaznacheeva T.V., Demidova E.Y., Berseneva V.V. Molecular mechanisms responsible for the impact of antiepileptic therapy on bone mineral density of epileptic patients. Nevrologiya, neiropsikhiatriya, psikhosomatika / Neurology, Neuropsychiatry, Psychosomatics. 2016; 1S: 59–65 (in Russ.). http://doi.org/10.14412/2074-2711-2016-1S-59-65.]
17. Li Q., Zhang Z., Fang J. Hormonal changes in women with epilepsy. Neuropsychiatr Dis Treat. 2024; 20: 373–88. https://doi.org/10.2147/NDT.S453532.
18. Bauer J., Stefan H., Schrell U., et al. Neurophysiologic principles and clinical value of post-convulsive serum prolactin determination in epileptic seizure. Fortschr Neurol Psychiatr. 1989; 57 (11): 457–68 (in German). https://doi.org/10.1055/s-2007-1001142.
19. Panahi Y., Fathi E., Shafiian M.A. The link between seizures and prolactin: a study on the effects of anticonvulsant medications on hyperprolactinemia in rats. Epilepsy Res. 2023; 196: 107206. https://doi.org/10.1016/j.eplepsyres.2023.107206.
20. Wang M.F. Effect of seizures and antiepileptic drugs on prolactin secretions. Di Yi Jun Yi Da Xue Xue Bao. 2002; 22 (8): 742–4.
21. Zhang Y.X., Shen C.H., Lai Q.L., et al. Effects of antiepileptic drug on thyroid hormones in patients with epilepsy: a meta-analysis. Seizure. 2016; 35: 72–9. https://doi.org/10.1016/j.seizure.2016.01.010.
22. Mohamed W.S., Nageeb R.S., Hashim N.A., Omran A.A. Effect of valproate versus levetiracetam monotherapy on reproductive functions in newly diagnosed epileptic males. Egypt J Neurol Psychiatr Neurosurg. 2019; 55 (1): 1–6. https://doi.org/10.1186/s41983-0190088-5.
23. Xiaotian X., Hengzhong Z., Yao X., et al. Effects of antiepileptic drugs on reproductive endocrine function, sexual function and sperm parameters in Chinese Han men with epilepsy. J Clin Neurosci. 2013; 20 (11): 1492–7. https://doi.org/10.1016/j.jocn.2012.11.028.
24. Shih F.Y., Chuang Y.C., Chuang M.J., et al. Effects of antiepileptic drugs on thyroid hormone function in epilepsy patients. Seizure. 2017; 48: 7–10. https://doi.org/10.1016/j.seizure.2017.03.011.
About the Authors
N. A. SivakovaRussian Federation
Natalia A. Sivakova, PhD
3 Bekhterev Str., Saint Petersburg 192019
WoS ResearcherID: I-7015-2018
I. V. Abramova
Russian Federation
Irina V. Abramova
3 Bekhterev Str., Saint Petersburg 192019
I. Yu. Trukhina
Russian Federation
Irina Yu. Trukhina
3 Bekhterev Str., Saint Petersburg 192019
V. P. Rybasova
Russian Federation
Varvara P. Rybasova
3 Bekhterev Str., Saint Petersburg 192019
E. D. Kasyanov
Russian Federation
Evgeny D. Kasyanov, PhD
3 Bekhterev Str., Saint Petersburg 192019
Scopus Author ID: 57205549541
L. V. Lukina
Russian Federation
Larisa V. Lukina, PhD
3 Bekhterev Str., Saint Petersburg 192019
Scopus Author ID: 16520904200
V. A. Mikhailov
Russian Federation
Vladimir A. Mikhailov, Dr. Sci. Med.
3 Bekhterev Str., Saint Petersburg 192019
WoS ResearcherID: B-3272-2017
G. E. Mazo
Russian Federation
Galina E. Mazo, Dr. Sci. Med.
3 Bekhterev Str., Saint Petersburg 192019
Scopus Author ID: 6603942525
Review
For citations:
Sivakova N.A., Abramova I.V., Trukhina I.Yu., Rybasova V.P., Kasyanov E.D., Lukina L.V., Mikhailov V.A., Mazo G.E. Anticonvulsants impacting bone metabolism: interim results from a cross-sectional study. Epilepsy and paroxysmal conditions. 2024;16(3):192–201. https://doi.org/10.17749/2077-8333/epi.par.con.2024.202
JATS XML

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.







































