Scroll to:
Stimulus-induced periodic discharges in response to low-frequency photostimulation in a female patient in recovery period after middle cerebral artery stroke
https://doi.org/10.17749/2077-8333/epi.par.con.2023.155
Abstract
Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs) represent a relatively common phenomenon recorded during a long-term electroencephalogram (EEG) monitoring allowing to capture the altered state and response to auditory, tactile or nociceptive stimulation in critically ill patients. It is a nosologically non-specific phenomenon, and its relation to ictal event remains debated. We present a clinical case in which SIRPIDs were recorded in the affected dominant hemisphere in response to low-frequency photostimulation in a 60-years-old woman recovering after middle cerebral artery stroke. No ictal events were recorded during routine EEG monitoring; the patient was not in critical condition.
Keywords
For citations:
Kanshina D.S., Okuneva I.V., Surma M.A., Bronov O.Yu., Nikitin S.S. Stimulus-induced periodic discharges in response to low-frequency photostimulation in a female patient in recovery period after middle cerebral artery stroke. Epilepsy and paroxysmal conditions. 2023;15(3):275–281. https://doi.org/10.17749/2077-8333/epi.par.con.2023.155
INTRODUCTION / ВВЕДЕНИЕ
Stimulus-induced, rhythmic, periodic or ictal dis- charges (SIRPIDs) represent an electrographic pattern that occurs in up to 12% of cases during long-term electroencephalographic (EEG) monitoring of critical ill patients being exerted as an altered state in response to diverse auditory, tactile, and nociceptive stimuli [1–3]. It is a nosologically nonspecific phenomenon described in hemorrhages, posthypoxic, posttraumatic, toxic-metabolic, infectious brain damage, and prion diseases [1]. It must be emphasized that all patients, described in the publications were in critical condition [3].
A series of studies established the relationship between recorded SIRPIDs and spontaneous electrographic seizures, despite that neuroimaging verifying a rise in cerebral blood flow within the zone of pattern generation upon recording was not obtained [4]. Currently, the major theory in SIRPIDs generation relies on disruption of thalamo-cortical projections in an abnormally or hyperexcitable area of the cerebral cortex [1][2]. Small population-based studies found no correlation between sex, age, history of epilepsy, background rhythm reactivity, and SIRPIDs recording [3]. An opportunity of recording periodic lateralized discharges
(PLDs) induced by low-frequency photostimulation was described in a non-critical patient with paroxysmal dystonic choreoathetosis in the hemisphere contralateral to motor paroxysms [5].
CASE REPORT / КЛИНИЧЕСКИЙ СЛУЧАЙ
In 2020, 14 months after suffering a hemorrhagic stroke in the left hemisphere of the brain with the formation of an intracerebral hematoma with a breakthrough into the ventricular system of the brain a 60-year-old woman underwent rehabilitation treatment at Pirogov National Medical and Surgical Center. In the acute period, trepanation of the skull and removal of an intracerebral hematoma of the left frontotemporal region was performed. Medical care was provided in full compliance with the standards and clinical guidelines, the patient filled out a form of voluntary informed consent during hospitalization.
Ethical aspects / Этические аспекты
All medical care was provided in full compliance with the standards, procedures and clinical recommendations, as well as the principles of the Helsinki Declaration of the World Medical Association (Fortaleza, Brazil, 2013). During hospitalization, the patient filled out a form of voluntary informed consent permitting the use of obtained data for scientific purposes.
Neurological status / Неврологический статус
Right-sided hemiparesis with minimal movements in the upper limb; in the lower limb proximal sections 2.5–3 points according to the Medical Research Council Weakness Scale (MRC); in the foot, minimal movements were recorded. Muscle tone in the paretic limbs was moderately increased according to the spastic type (1 point on the Ashfort scale), right-sided hemihypesthesia, sensory-motor aphasia, magnitude according to the Rankin scale was 3 points.
Rehabilitation methods / Методы реабилитации
The treatment was carried out according to an individual medical rehabilitation plan, including sessions of neuropsychological rehabilitation, transcranial magnetic stimulation, speech restoration sessions, therapeutic exercises, individual mechanotherapy in the biofeedback mode, classes on a robotic complex for the upper limbs as well as classes on a robotic complex Lokomat (Hocoma AG, Switzerland).
EEG / ЭЭГ
During hospitalization, a standard EEG was performed according to the approved protocol [6][7] by using the Neuron-spectrum 3 electroencephalograph (Neurosoft, Russia). Recording parameters were as follows: double banana montage, sweep 30 mm/s, sensitivity 7 µV/div, high pass filter 0.5 Hz, low pass filter 70 Hz.
During the test with 1 Hz photostimulation, stimulusinduced lateralized periodic discharges were recorded in the affected hemisphere outside the ictal event. Registration of the background EEG in the state of passive wakefulness was accompanied by arising transient delta slowdown, lateralized according to the zone of structural changes (Fig. 1).

Figure 1. Patient Z. Electroencephalographic recording fragments
(background recording, double banana montage, sweep 30 mm/sec,
sensitivity 7 µV/div, high-pass filter 0.5 Hz, low-pass filter 70 Hz):
a – in passive wakefulness state, continued lateralized
left hemispheric delta deceleration was registered;
b – 1 Hz rhythmic photostimulation, lateralized left hemispheric discharges
of three-phase morphology were registered (highlighted by arrows and brackets),
the frequency of discharges corresponds to the frequency of photostimulation
Рисунок 1. Фрагменты электроэнцефалографической записи пациентки З.
(фоновая запись, монтаж double banana, развертка 30 мм/с,
чувствительность 7 мкВ/дел, фильтр высоких частот 0,5 Гц,
фильтр низких частот 70 Гц):
а – регистрация в состоянии пассивного бодрствования,
преходящее латерализованное по левому полушарию дельта-замедление;
b – фотостимуляция с частотой 1 Гц,
стимул-индуцированные ритмичные периодические латерализованные
левополушарные разряды выделены стрелками и скобками,
частота разрядов соответствует частоте фотостимуляции
MRI / МРТ
Brain neuroimaging was performed by using a magnetic resonance tomograph Magnetom Aera (Siemens, Germany) with a magnetic field induction of 1.5 T. A standard protocol was applied including the following sequences: T1 weighted image (T1 WI) 3D, T2-WI in the axial plane, T2 FLAIR (fluid attenuated inversion recovery) in the coronal plane, SWI (susceptibility) weighted imaging) in the axial plane as well as the diffusion-weighted image (diffusion-weighted image, DWI) (b=0, b=1000) and the measured apparent diffusion coefficient (ACD). Neuroimaging revealed an extensive area of structural changes with prolapse of the brain tissue in the area of the postoperative bone defect (Fig. 2).
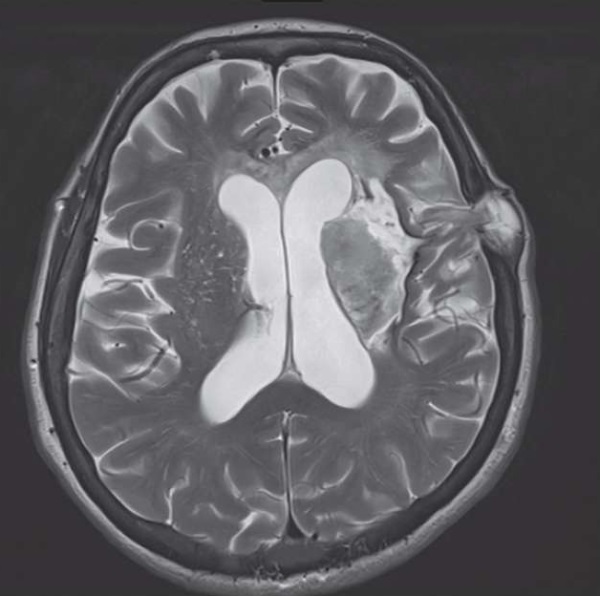
Figure 2. Patient Z. Axial T2-WI magnetic resonance image.
Cystic-glial (with hemorrhagic component) changes in the left frontal lobe, thalamus,
internal capsule, lentiform nucleus, putamen, insula and external capsule.
Small portion of brain tissue herniated through the postsurgical bone defect
Рисунок 2. Магнитно-резонансная томограмма пациентки З.,
режим T2-WI, аксиальный срез. Кистозноглиозные (с геморрагическим компонентом)
изменения лобной доли левого полушария головного мозга, области таламуса,
скорлупы и чечевицеобразного ядра, островка, внутренней и наружной капсулы.
Определяется послеоперационный костный дефект
с небольшим пролабированием мозговой ткани
DISCUSSION / ОБСУЖДЕНИЕ
While searching the Pubmed database with the query of the keyword SIRPIDs, 33 papers were retrieved, the vast majority of which are devoted to recording of this phenomenon in critically ill patients. SIRPIDs was first described in 2004 by L.J. Hirsh et al. as a reproducible phenomenon recorded in response to sound or physical stimulation in patients in critical condition [8].
According to the standardized terminology proposed by the American Society of Clinical Neurophysiology (ACNS), the following SIRPIDs electrographic patterns are distinguished: rhythmic delta activity (stimulus-induced rhythmic delta activity, SI-RDA), periodic discharges (SI-PD), spike-wave complexes (SI-SW), ictal patterns (SI-seizures), bursts (SI-bursts), ictal interictal continuum (SI-IIC), and brief ictal rhythmic discharges (SI-BIRDs) [9].
A series of studies revealed the relationship between SIRPIDs localization and the area of brain damage [10]. At the same time, no data confirming the presence or absence of the relationship between the stimulus modality and stimulus modality-matching cortical association area was found.
According to the current theories of pathogenesis, thalamocortical projections transmitting afferent stimulation are topographically organized in the middle layers of specific cortical areas, whereas separate groups of thalamic neurons with a more diffuse cortical representation resulting in arising thalamo-cortico-thalamic network were observed [11]. On the one hand, the lateralization spread of the phenomenon may be accounted for by indirect inhibition of the thalamic reticular nucleus followed by excessive activation of cortical projections concomitant with subcortical damage. On the other hand, the emergence of SIRPIDs is coupled to lowered excitatory thalamocortical projections due to thalamus-specific structural damage. It seems interesting that SIRPIDs can be considered as a type of reactivity in the context of viable hyperexcitable cerebral cortex [12].
It is noteworthy, the presented clinical observation was found to describe SIRPIDs in response to low frequency (1 Hz) photostimulation in the non-critical patient at the time of recording. The lateralization of this phenomenon upon activating stimulus spreading from the occipital cortex to the projection of the thalamus followed by volumetric conduction along the thalamo-cortical connections of the affected hemisphere is of interest based on existing theories of the pattern-related pathogenesis.
The case of PLDs recorded after low-frequency photo- stimulation described so far in the patient with paroxysmal dystonic choreoathetosis in the contralateral side of motor paroxysms of the hemisphere confirmed the lack of structural changes in the area of phenomenon registration [5]. According to J.W. Lance, any disruption of the cortical control of the neostriatum and its thalamic connections via an unknown neurotransmitter mechanism can cause photosensitivity PLDs that may exist in a “latent” form; its morphology is mediated by a complex reflex mechanism [13].
No consensus on the need to treat patients with antiepileptic drugs if they are registered with SIRPIDs exists. Recent studies show conflicting data between induced rhythmic patterns and seizures [12].
Our observation proves an opportunity to record a rare pattern – a stimulus-induced rhythmic lateralized periodic discharges (SI-PLDs) during low-frequency photostimulation in the patient in the recovery period after middle cerebral artery (MCA) stroke (see Fig. 1, 2) in the affected hemisphere. The registered pattern corresponds to the developed ACNS criteria proposed for SIRPIDs being neither a photoparoxysmal response nor a visual evoked potential due to its delayed appearance in response to the presented stimulus, the morphology and amplitude of the complexes, and the recording area not being associated with the visual analyzer.
Acknowledgement / Благодарность
The authors are thankful to Amayak G. Brutyan, Head of the Laboratory of Clinical Neurophysiology at Scientific Center of Neurology (Russian Academy of Sciences) for discussion of the clinical case data.
CONCLUSION / ЗАКЛЮЧЕНИЕ
The presented clinical case demonstrated SI-PLDs registered in the non-critical patient during standard EEG of wakefulness in the post-MCA stroke recovery period associated with extensive damage to the dominant (left) hemisphere involving thalamic structures. In this case, the pattern was not ictal. Low-frequency photostimulation, an inducing test, can provoke recording periodic rhythmic lateralized discharges, including the area with prominent structural damage to thalamo-cortical projections.
References
1. Johnson E.L., Kaplan P.W., Ritzl E.K. Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges (SIRPIDs). J Clin Neurophysiol. 2018; 35 (3): 229–33. https://doi.org/10.1097/WNP.0000000000000434.
2. Fernández-Torre J.L., Martín-García M., Marco de Lucas E., Hernández-Hernández M.A. Asymmetric SIRPIDs in a vertebrobasilar stroke: implications for understanding its origin. Clin Neurol Neurosurg. 2020; 196: 105980. https://doi.org/10.1016/j.clineuro.2020.105980.
3. Van Straten A.F., Fesler J.R., Hakimi R., et al. SIRPIDs: prevalence and outcome in critically ill patients. J Clin Neurophysiol. 2014; 31 (5): 418–21. https://doi.org/10.1097/WNP.0000000000000094.
4. Zeiler S.R., Turtzo L.C., Kaplan P.W. SPECT-negative SIRPIDs argues against treatment as seizures. J Clin Neurophysiol. 2011; 28 (5): 493–6. https://doi.org/10.1097/WNP.0b013e318231c00a.
5. Jacome D.E., Risko M. Photic induced-driven PLEDs in paroxysmal dystonic choreoathetosis. Clin Electroencephalogr. 1984; 15 (3): 151–4. https://doi.org/10.1177/155005948401500306.
6. Dash D., Dash C., Primrose S., et al. Update on minimal standards for electroencephalography in Canada: a review by the Canadian Society of Clinical Neurophysiologists. Can J Neurol Sci. 2017; 44 (6): 631–42. https://doi.org/10.1017/cjn.2017.217.
7. Guidelines for carrying out of routine EEG of Neurophysiology Expert Board of Russian League Against Epilepsy. Epilepsia i paroksizmalʹnye sostoania / Epilepsy and Paroxysmal Conditions. 2016; 8 (4): 99–108 (in Russ.).
8. Hirsch L.J., Claassen J., Mayer S.A., Emerson R.G. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004; 45 (2): 109–23. https://doi.org/10.1111/j.00139580.2004.38103.x.
9. Hirsch L.J., Fong M.W.K., Leitinger M., et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021; 38 (1): 1–29. https://doi.org/10.1097/WNP.0000000000000806.
10. Benarroch E.E. The midline and intralaminar thalamic nuclei: anatomic and functional specificity and implications in neurologic disease. Neurology. 2008; 71 (12): 944–9. https://doi.org/10.1212/01.wnl.0000326066.57313.13.
11. Barbella G., Lee J.W., Alvarez V., et al. Prediction of regaining consciousness despite an early epileptiform EEG after cardiac arrest. Neurology. 2020; 94 (16): e1675–83. https://doi.org/10.1212/WNL.0000000000009283.
12. Rodriguez Ruiz A., Vlachy J., Lee J.W., et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017; 74 (2): 181–8. https://doi.org/10.1001/jamaneurol.2016.4990.
13. Lance J.W. Familial paroxysmal dystonic choreoathetosis and its differentiation from related syndromes. Ann Neural. 1977; 2 (4): 285– 93. https://doi.org/10.1002/ana.410020405.
About the Authors
D. S. KanshinaRussian Federation
Daria S. Kanshina – MD, PhD, Senior Researcher, Department of Functional Diagnostics
Moscow
I. V. Okuneva
Russian Federation
Irina V. Okuneva – Researcher, Clinical Neurophysiology Groups, Department of Emergency Neurosurgery
Moscow
M. A. Surma
Russian Federation
Maria A. Surma – Neurologist, Functional Diagnostician
Moscow
O. Yu. Bronov
Russian Federation
Oleg Yu. Bronov – MD, PhD, Chief of Chair of Radiation Diagnostics with a Course of Clinical Radiology, Institute of Advanced Medical Training; Radiologist
Moscow
S. S. Nikitin
Russian Federation
Sergey S. Nikitin – Dr. Med. Sc., Professor, Chief of Chair of Genetics of Neurological Diseases
Moscow
Review
For citations:
Kanshina D.S., Okuneva I.V., Surma M.A., Bronov O.Yu., Nikitin S.S. Stimulus-induced periodic discharges in response to low-frequency photostimulation in a female patient in recovery period after middle cerebral artery stroke. Epilepsy and paroxysmal conditions. 2023;15(3):275–281. https://doi.org/10.17749/2077-8333/epi.par.con.2023.155

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.










































