Scroll to:
Cognitive impairment in childhood-onset epilepsy
https://doi.org/10.17749/2077-8333/epi.par.con.2024.176
Abstract
In pediatric practice, epilepsy holds one of the leading places among neurological pathologies. Along with seizures, a child's intellectual impairment lowering quality of life plays a crucial role in social disintegration. Cognitive impairments occuring in idiopathic generalized epilepsies (IGE) and self-limited epilepsy with centrotemporal spikes (SeLECTS) considered benign have been widely investigated. However, available data suggest that such disorders result in multiple persistent alterations in the cognitive sphere. In this case, features of the epilepsy etiopathogenesis account for disease early onset and profoundly remodeled structures involved in the implementation of cognitive functions. Current review is aimed to summarizing data regarding developmental mechanisms and range of cognitive impairment in IGE and SeLECTS.
Keywords
For citations:
Paramonova A.I., Lysova K.D., Timechko E.E., Senchenko G.V., Sapronova M.R., Dmitrenko D.V. Cognitive impairment in childhood-onset epilepsy. Epilepsy and paroxysmal conditions. 2024;16(1):54-68. https://doi.org/10.17749/2077-8333/epi.par.con.2024.176
INTRODUCTION / ВВЕДЕНИЕ
The incidence of epilepsy in the pediatric population ranges from 0.5% to 2% [1][2]. Currently, much attention has been paid to the epilepsy-associated cognitive-behavioral problems [3]. According to the available studies, cognitive impairment occurs in children with epilepsy in 20–60% of cases and 4.8 times more often than in the general population [4][5]. In some prospective studies, patients with severe cognitive impairments had the lowest chance of achieving long-term seizure remission and an increased risk of early death [6].
The simultaneous disorganization of several epilepsy-related intellectual functions leads to lowered overall social integration in children and additionally directly affects the educational process. According to the latest data, up to 50% of pediatric patients have learning problems, including those with average IQ scores [7].
Taking into account the anatomical and physiological characteristics of brain ontogenesis, the influence of epileptogenic processes during childhood can result in neuropsychic development disorders. Early-onset epilepsy in two out of three cases is characterized by impaired general cognitive abilities, adaptive behavior and social functions [8]. In the study by Ž. Rogač et al. (2022), intellectual and emotional-volitional disorders in children were identified within the first six months after seizures onset [9]. Other data indicate that already at the onset of the disease about 30% of children have behavioral and cognitive impairments [10].
Self-limited epilepsy with centrotemporal spikes (SeLECTS) [11] is one of the most common forms of childhood epilepsy. SeLECTS is diagnosed in 10–20% of cases. For SeLECTS, the concept of benignity can be fully attributed only to seizures outcome, because studies demonstrate the presence of persistent neuropsychological disorders in 28–53% of patients [12].
Idiopathic generalized epilepsies (IGEs) occur in 15–20% of new cases and are also classified as benign forms [13]. According to the classification proposed by the International League Against Epilepsy (ILAE), IGEs were identified as a separate subgroup of genetic generalized epilepsies, taking into account the high prevalence, similar clinical course and electroencephalographic (EEG) patterns, the potential for transition from one form to another as well as a relatively favorable outcome [11][14]. However, such patients are also characterized by neuropsychological disorders that persist or progress into adulthood [15].
Obje с tive: to summarize publications on the mechanisms of developing cognitive impairment in IGEs and SeLECTS and to compare a relevant clinical spectrum.
MATERIAL AND METHODS / МАТЕРИАЛ И МЕТОДЫ
A search for publications was carried out in PubMed/MEDLINE, ScienceDirect and Google Scholar databases over the past 10 years available in Russian and English languages (Fig. 1) using the following query terms: “childhood epilepsy”, “idiopathic generalized epilepsy”, “childhood absence epilepsy”, “juvenile absence epilepsy”, “juvenile myoclonic epilepsy”, “self-limited epilepsy with centrotemporal spikes” in combination with separate keywords: “cognitive impairments”, “cognitive functions”, “neuropsychological testing”. The search was also repeated using common disease name abbreviations (eg., IGE, CAE, JAE, JME) and names revised in the 2017 and 2022 ILAE classifications.
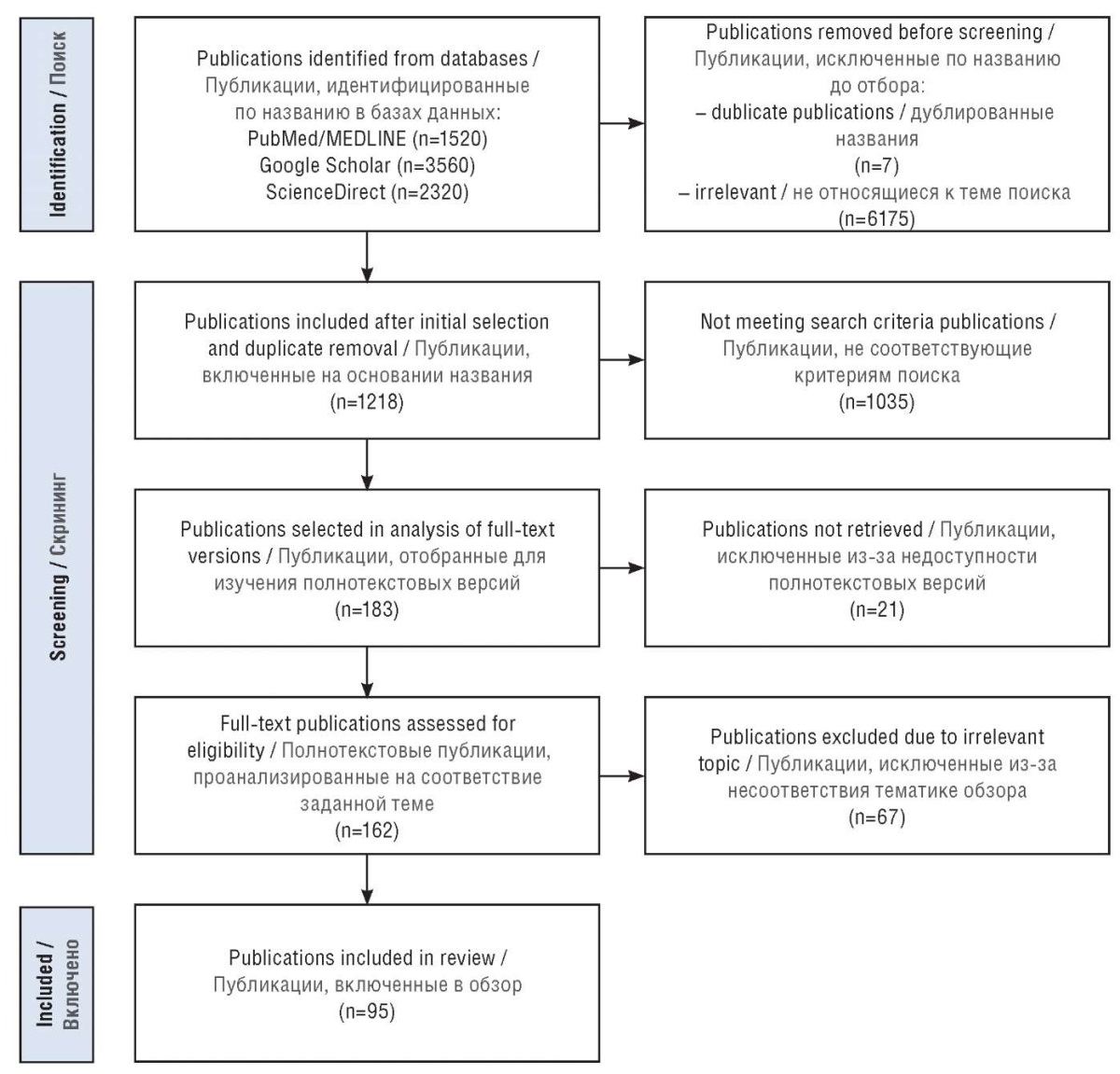
Figure 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) workflow
Рисунок 1. Блок-схема PRISMA
(англ. Preferred Reporting Items for Systematic reviews and Meta-Analyses)
Review articles and original studies were analyzed, and duplicate articles were excluded. Some earlier works were also reviewed and manually selected from the reference lists retrieved from review articles. The selection of publications was carried out by two researchers independently of each other, involving a third researcher in case of opinions divergence.
The review did not include articles on epilepsy with generalized tonic-clonic seizures alone.
RESULTS AND DISCUSSION / РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
Characteristics of cognitive impairment in IGEs and SeLECTS / Характеристика когнитивных нарушений при ИГЭ и SeLECTS
According to the current data, disorders of neuropsychological functions in patients with epilepsy are heterogeneous and can affect several brain areas at once: memory and learning, executive function, speech, visual perception, constructive thinking and emotional intelligence [16].
According to the original studies and systematic reviews, the neuropsychological profile of disorders in IGE patients of different ages is characterized by simultaneous damage to several cognitive domains [17], including executive, perceptual and visuospatial functions, verbal generativity and non-verbal thinking, stability of attention, speed of information processing and working memory [18][19].
Children are characterized by decreased academic performance and arithmetic skills, reading and writing [20][21], which also aligns with the available data on impaired executive functions and decreased attention in IGE children [22].
In accordance with the severity of clinical manifestations, three IGE cognitive phenotypes were identified:
- no impairment, with average neuropsychological test results consistent with healthy controls (44%);
- mild impairment in several cognitive areas (44%);
- alterations in all cognitive domains with severe attention impairment (12%).
In this case, no relation between severity of clinical manifestations and a specific IGE syndrome is observed; the greatest impact is exerted by factors such as family history (parental IQ), developmental disorders in early childhood, and the detected changes in neuroimaging data [19].
Childhood absence epilepsy (CAE) is characterized by a wide range of alterations including deteriorated verbal and non-verbal memory, visual attention, speech impairment and executive function along with normal or slightly reduced IQ levels [23]. Impairments in behavioral inhibition, psychosocial functioning, and motor function are also often detected [24]. The combination of characteristic changes suggest a complex damage with predominant dysfunction in the frontal lobes [25]. At the same time, it has been evidenced about persistent attention deficit in СAE children both before treatment and during compensation of seizures [26], which indicates that the frontal cortex is primarily involved in the general pathogenesis of the disease.
The spectrum of cognitive impairment in juvenile absence epilepsy (JAE) is also associated with frontal lobe dysfunction [27]. In a study of intellectual profile among IGE adult patients, the JAE/CAE group with persistent seizures revealed the lowest IQ level and the most noticeable impairments in executive function, learning and information acquisition [18][24].
Children with JME are characterized by multiple cognitive impairments [27]. E.H. Kim et al. (2016) note a slightly decreased IQ level, disturbances in attention, control of reaction inhibition, verbal and working memory as well as the speed and quality of information processing and flexibility along with fluency of speech [28].
Research by D.N. Almane et al. (2019) has also demonstrated worsening results in all cognitive domains tested; in most cases, neuropsychological problems arose before the onset of epilepsy [29]. In a prospective study on the development of cognitive functions in JME children vs. with healthy subjects, impairments were identified both at the time of seizures onset and 2 years after disease onset, at the same time, improved skills were noted in the study group, but the level and speed did not correspond to the average range among healthy children [22].
Children with SeLECTS are known to have a higher risk of cognitive, behavioral or emotional difficulties [30]. Most systematic reviews indicate impairments in memory, attention, visuospatial and executive functions [31]. It was also noted that the average IQ level is lower in children with SeLECTS. Many researchers identify common disorders of verbal function, including changes in both expressive and receptive speech with impaired semantic language processing [32]. It is typical that the risk of developing speech dysfunction increases in parallel with longer disease duration [33].
In addition to impairments in verbal domain, it has been increasingly evidenced about altered memory functions in children with SeLECTS. At the same time, some studies indicate a deteriorated memorization of both verbal and nonverbal material [31][34]. According to other data, the majority are presented as disorders of predominantly verbal memory [35], which, according to some studies, is consistent with the predominant deficit of speech functions and is secondary by nature due to altered processes of verbal encoding and storage of information [31].
Etiological factors / Этиологические факторы
Modern ideas about the pathogenesis of epilepsy suggest a multifactorial nature of cognitive impairment (Fig. 2), primarily coupled to the three most important predictors [17]:
- etiology considered the most important cause;
- the nature and frequency of seizures;
- effects of antiepileptic therapy.
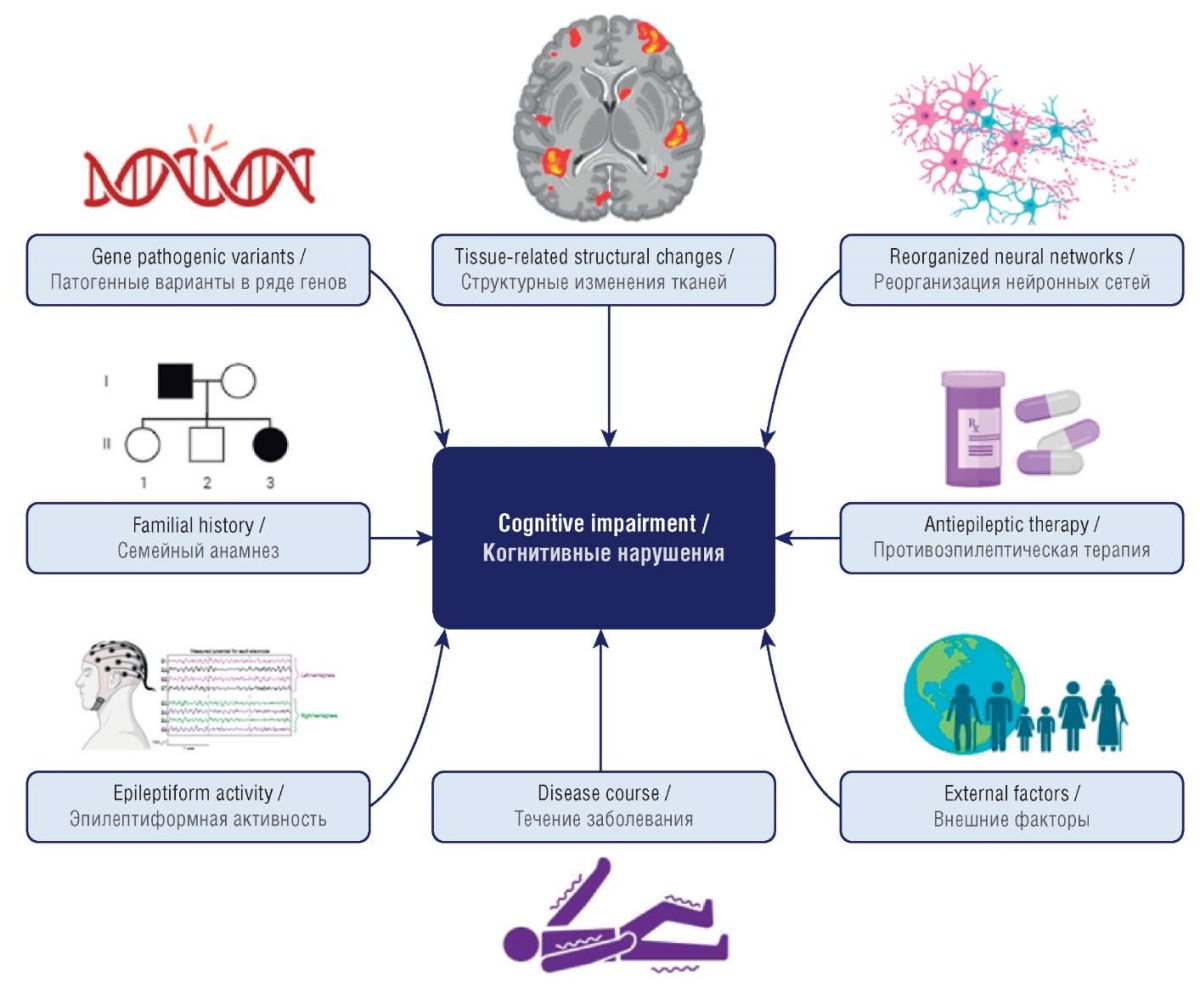
Figure 2. Factors involved in development of epilepsy-related cognitive impairment
(drawn by authors)
Рисунок 2. Факторы развития когнитивных нарушений при эпилепсии
(рисунок авторов)
Genetic predictors / Генетические предикторы
The presence of clinical and anamnestic similarity between CAE, JAE and JME implies common etiopathogenetic mechanisms in development of such IGE forms [11][14]. General genetic factors regulating epileptogenesis are discussed in numerous studies; in particular, there are the data on an opportunity for all four IGE syndromes to occur in a single family [36]. In addition, study data demonstrate similarities in the structural and functional characteristics of EEG and magnetic encephalography in patients and healthy siblings [37].
The general mechanisms underlying development of concomitant cognitive impairment are also discussed. The genetic etiology of neuropsychological dysfunction is indicated by the manifestation of similarly altered cognitive profile both in patients with IGE and related relatives [36].
Patients with SeLECTS and their siblings also show change in similar cognitive domains. The genetic nature is corroborated by the studies on intellectual function, demonstrating a specific cognitive phenotype with impaired language functions, verbal memory and attention in both children with SeLECTS and healthy siblings [38].
Genome-wide studies demonstrate a polygenic mode of inheritance and identified multiple single nucleotide polymorphisms accounting for about 58% of genetic changes in IGE [39]. Changes in the gamma-aminobutyric acid receptor genes GABRA1 (rs2279020), GABRG2 (rs121909673), GABRA6 (rs3219151) are commonly recognized [40–42]. According to genetic studies, the role of genes encoding calcium channels, CACNA1H, CACNA1G and CACNG3, has been shown in the etiopathogenesis of CAE [43][44].
In patients with JME, several individual loci are involved, of which GABRA1, GABRD, EFHC1, BRD2, CASR and ICK are considered the most pathogenic, displaying complex inheritance [45, 46]. The T-allele in the GJD2 gene encoding the connexin 36 protein and increasing the risk of epileptogenesis has been also discussed [47][48]. The identified genetic variants, however, are not the only sufficient etiological factor for the development of IGE and concomitant cognitive deficits. The cumulative and synergistic effect as well as epigenetic and environmental factors are of great importance [14][39].
According to a study by W. Xiong and D. Zhou (2017), up to 59% of patients with SeLECTS had a positive family history [49]. SeLECTS is also characterized by polygenic inheritance, with pathogenic variants in several genes reported: GRIN2A, KCNQ2, KCNQ3, DEPDC5, ELP4-PAX6, GABAA-R, GABRG2, RBFOX1/3, RYR2, CHRNA5 and KALRN [50–52]. It was evidenced that loci 16p12–11.2, 15q14 and 11p13 support the hereditary nature of centrotemporal spikes. At the same time, pleiotropy for speech dyspraxia and spikes was revealed for the 11p13 locus. The 16p11.2 microduplication is considered characteristic of SeLECTS because it was detected both in isolated spikes and patients with seizures [49].
Disease course and antiepileptic therapy effects / Характер течения заболевания и эффекты противоэпилептической терапии
The severity of the disease with persistent seizures is considered a crucial factor in developing cognitive impairment. For instance, A.W. Byars et al. (2014) in the study with 193 sick children showed a correlation between the nature of epilepsy course and the severity of affected speech function, regardless of the etiology and disease form. The most unfavorable data at the time of disease onset and 1.5 years later were shown in children with persistent seizures compared to children with intermittent seizures and healthy individuals [53].
Results from a 50-year-long prospective study on cognitive outcomes in patients with childhood-onset epilepsy also demonstrated an association between cognitive decline and persistent seizures. Patients with active epilepsy receiving antiepileptic drugs (AEDs) vs. those in remission had clinically significant impairments according to the neuropsychological testing data [15].
The development of cognitive impairment in patients receiving AEDs has been actively discussed. Among children with various forms of epilepsy, decreased attention and slow thinking are the most common AED-related side effects [54]. Study by I.N. Mohamed et al. (2018) showed that 1/3 of children had a correlation between a reduced IQ level and treatment with more than one AEDs [55]. According to some data, AEDs rather than etiology and course of epilepsy stronger affect the severity of psychobehavioral dysfunction [56].
Brain structural alterations / Структурные изменения головного мозга
Despite the fact that the described types of epilepsy are classified as forms lacking brain structural pathology, neuroimaging data indicate persistent anatomical alterations (Table 1).
Table 1. Brain structural alterations in idiopathic generalized epilepsies
and self-limited epilepsy with centrotemporal spikes (SeLECTS)
Таблица 1. Структурные изменения головного мозга
при идиопатических генерализованных эпилепсиях
и самокупирующейся эпилепсии с центротемпоральными спайками
(англ. self-limited epilepsy with centrotemporal spikes, SeLECTS)
|
CAE/JAE // ДАЭ/ЮАЭ |
JME / ЮМЭ |
SeLECTS |
|
– Decreased gray matter volume in the frontal lobe / Уменьшение объема серого вещества лобной доли – Decreased gray matter volume in the right anterior temporal region / Уменьшение объема серого вещества правой передней височной области – Decreased gray matter volume in the cerebellum / Уменьшение объема серого вещества мозжечка – Decreased basal ganglia volume (caudate nucleus and putamen) / Уменьшение объема базальных ганглиев (хвостатое ядро и скорлупа) – Increased gray matter volume in the thalamus / Увеличение объема серого вещества таламусов |
– Decreased gray matter volume in the frontal lobe / Уменьшение объема серого вещества лобной доли – Decreased gray matter volume in the posterior cingulate cortex / Уменьшение объема серого вещества задней области поясной извилины – Decreased volume of the supplementary motor area cortex / Уменьшение объема коры дополнительной двигательной области – Lowered functional activity in the corpus callosum / Снижение функциональной активности в мозолистом теле – Decreased basal ganglia volume (caudate nucleus, putamen) / Уменьшение объема базальных ганглиев (хвостатое ядро, скорлупа) – Decreased white matter density / Снижение плотности белого вещества |
– Decreased total frontal lobe volume / Уменьшение общего объема лобных долей – Thickened right frontotemporal cortex / Утолщение правой лобно-височной коры – Thickened left orbitofrontal cortex / Утолщение орбитофронтальной коры слева – Thickened bilateral precentral gyrus cortex / Утолщение коры прецентральных извилин с двух сторон – Bilaterally increased mass and volume of the putamen and amygdala / Двустороннее увеличение массы и объема скорлуп и миндалевидных тел |
Note. CAE – childhood absence epilepsy;
JAE – juvenile absence epilepsy;
JME – juvenile myoclonic epilepsy.
Примечание. ДАЭ – детская абсансная эпилепсия;
ЮАЭ – юношеская абсансная эпилепсия;
ЮМЭ – юношеская миоклоническая эпилепсия.
In the study by J.J. Lin et al. (2014) with JME children, disorders of age-related brain development were found in downmodulating age-associated processes of lowered gray matter volume and thickness in the fronto-parietal-temporal regions [22]. There are experimental data regarding the impact of epileptogenesis in JME on regulating developmental events in the cerebral cortex. A mouse study revealed a neuronal migration disorder associated with the EFHC1 gene [22][61].
Features of neural networks organization and functioning / Особенности организации и функционирования нейронных сетей
Another important etiopathogenetic mechanism behind cognitive dysfunction is coupled to altered neural networks in epilepsy [62]. According to the concept of network organization, epilepsy can be considered as a pathology with disrupted cerebral networks as well as altered interplay and mutual regulation in related brain areas due to epileptogenesis [63]. Research data demonstrate an abnormal structure of brain networks and nodes that ensure multiple complex inter-network crosstalk that can be directly involved in production and transmission of pathological impulses in epilepsy [62][64].
The study by T.S. Kellermann et al. (2015) in children with epilepsy, showed that the organization of networks was characterized by less differentiated and impaired connections between domains of some functions with the rest of the global network as well as relative isolation of domains of attention and executive function. Neural networks in children without epilepsy and neuropsychological development disorders had a clear modular organization [65]. According to other sources, patients with epilepsy had disturbances in the temporal and spatial variability of the “default mode” networks (brain passive mode networks) including the posterior cingulate gyrus, prefrontal cortex, middle and superior frontal cortex, anterior cingulate and angular gyri, were identified [12][66].
Patients with IGE have been described to have changed structural and functional characteristics in neural networks including disrupted integrability and isolation of affected functional cognitive centers, restructuring of “default mode” networks as well as increased spatiotemporal networks variability [63][66][67].
In CAE, the most prominent changes in the networks of the frontal lobe region have been identified including dorsolateral prefrontal circuits and connections between the orbitofrontal and motor and premotor areas associated with the implementation of executive and speech functions [67]. Among these patients, functional impairments in the attention network were also identified with altered signal from the insula, frontal operculum, and medial frontal cortex [68]. Disrupted functional activity in the medial regions of the frontal cortex is also typical to JME. In addition, bilateral structural disorders in thalamo-frontal connections were also revealed in JME patients.
Patients with SeLECTS also exhibit extensive systemic brain disruption characterized by involvement and dysfunction in the areas outside the typical epileptogenic focus [66][69]. Some evidence suggests persistent abnormal functional interactions between cortical and subcortical structures regardless of detected seizures and epileptiform activity [70].
Multiple changes in language networks occur with high frequency among patients with SeLECTS and are characterized by impaired integration between the motor and language systems [64][71]. Studies demonstrate a decrease in impulse transmission from the epileptogenic zone, which includes the sensorimotor area, to Broca's center [72]. In addition, this pathology is also linked to changed activity in the basal ganglia as well as reshaped connections between the hippocampus and the ventral Brodmann area responsible for the regulation of language syntax [33][64][73].
The impact of epileptiform activity / Влияние эпилептиформной активности
According to functional studies, some patterns of neuropsychological changes have been identified depending on the nature of epileptiform activity in SeLECTS. Thus, children with unilateral vs. bilateral epileptiform spikes have a higher IQ level [74]. The study by A.E. Vaudano et al. (2019) has determined the negative impact of interictal epileptiform activity on speech networks functioning [75]. Another study revealed a detailed relationship between language dysfunction and spike localization, with phonology and speech production being more affected in the left-sided spikes, whereas right-sided localization of epileptiform activity had a greater impact on visuospatial skills and language processing [76].
The influence of interictal epileptiform activity during the NREM1 sleep phase on memory consolidation processes has also been demonstrated [34]. Similar data were obtained by J. Zhang et al. (2020), revealing impaired memory, reaction speed, and visuospatial functions in children with bilateral localization of epileptiform activity and confirming higher risk of cognitive deficits with a high activity index during the NREM sleep phase [77].
In children with EEG-detected centrotemporal spikes were found to have increased connections between the auditory and the somatomotor networks, which may be a compensatory mechanism for speech dysfunction [78].
For IGE, interictal epileptiform activity is also of importance. Patients with JME have more severe cognitive impairment while maintaining long-term generalized epileptiform activity [79]. The worst cognitive tests is typical for JME patients with asymmetric interictal generalized epileptiform activity [80].
Among patients with JAE, a link between prolonged epileptiform activity (more than 3 sec) and deteriorated information processing and memory was also noted [81]. The relationship between severe attention disorders and epileptic attack duration at the onset of CAE of more than 20 sec-long based on EEG data before the start of therapy has been shown [82].
Comparative profile of cognitive impairment in IGE and SeLECTS / Сравнительный профиль когнитивных нарушений при ИГЭ и SeLECTS
Current data are characterized by a marked heterogeneity in both cognitive-behavioral and functional, neuroimaging and clinical characteristics in epilepsy patients, which suggests a multifactorial etiology and pathogenesis of comorbid cognitive disorders [83]. The cognitive profiles of patients with different IGE syndromes display no clear differentiation [17]. When compared (Table 2), the multiplicity of lesions in IGE and SeLECTS is clearly visible with the majority of domains matching.
Table 2. Comparative characteristics of cognitive impairment
in idiopathic generalized epilepsies
and self-limited epilepsy with centrotemporal spikes (SeLECTS)
Таблица 2. Сравнительная характеристика когнитивных нарушений
при идиопатических генерализованных эпилепсиях
и самокупирующейся эпилепсии с центротемпоральными спайками
(англ. self-limited epilepsy with centrotemporal spikes, SeLECTS)
|
Cognitive impairment / Когнитивные нарушения |
Epilepsy form-specific distribution / Распределение по формам эпилепсии |
|
|
Speech functions / Речевые функции |
||
|
semantic speech perception / семантическое восприятие речи |
– |
SeLECTS |
|
phonemic speech analysis / фонематический анализ речи |
– |
|
|
expressive speech / экспрессивная речь |
JME / ЮМЭ |
|
|
writing and reading / письмо и чтение |
– |
|
|
Executive function / Исполнительные функции |
CAE, JAE, JME / ДАЭ, ЮАЭ, ЮМЭ |
SeLECTS |
|
Visual-spatial analysis / Зрительно-пространственный анализ |
– |
SeLECTS |
|
Attention / Внимание |
CAE, JAE, JME / ДАЭ, ЮАЭ, ЮМЭ |
SeLECTS |
|
Memory / Память |
||
|
verbal component / вербальный компонент |
CAE, JME / ДАЭ, ЮМЭ |
SeLECTS |
|
non-verbal component / невербальный компонент |
CAE, JАE / ДАЭ, ЮАЭ |
|
|
working memory / рабочая память |
JME / ЮМЭ |
– |
|
Regulated reaction inhibition / Регуляция торможения реакций |
CAE, JME / ДАЭ, ЮМЭ |
– |
|
Speed of thought and action / Скорость мышления и реакций |
CAE, JAE, JME / ДАЭ, ЮАЭ, ЮМЭ |
– |
|
Motor functions / Моторные функции |
CAE / ДАЭ |
SeLECTS |
|
Lowered IQ level / Снижение уровня IQ |
CAE, JAE, JME / ДАЭ, ЮАЭ, ЮМЭ |
SeLECTS |
Note. JME – juvenile myoclonic epilepsy;
CAE – childhood absence epilepsy; JAE – juvenile absence epilepsy.
Примечание. ЮМЭ – юношеская миоклоническая эпилепсия;
ДАЭ – детская абсансная эпилепсия; ЮАЭ – юношеская абсансная эпилепсия.
Research data agree that the determining profile of cognitive impairment in addition to response to therapy is affected by the location of the epileptogenic zone, age of onset, environmental factors and additional undifferentiated genetic factors.
Anatomical and physiological features in developing brain during epileptogenesis / Анатомо-физиологические особенности формирования головного мозга на фоне эпилептогенеза
The existing relationship between the age of disease onset and the development of neuropsychological disorders can be accounted for by development of cognitive and social skills during epileptogenesis.
Normally, the spatiotemporal organization in developing brain is characterized by general dynamic changes in gray matter volume and cortical thickness during childhood progression, reaching a maximum during puberty that gradually declines in adolescence. White matter volume increases within the first few years of life and continues to expand linearly in the parietofrontotemporal regions with the onset of puberty, which correlates with the formation of areas in the most complex higher cortical functions [84].
Also, a characteristic feature is the correspondence between morphometric changes in gray matter and the events of psychomotor development in childhood. At early age, primary involvement of the sensorimotor cortex is typical; at older age, the development of secondary and multimodal areas associated with the implementation of complex psychosocial skills predominates [85].
The latter occurs in the cortex and pathways of the frontal and temporal lobes involved in the implementation of speech and executive functions during physiological maturation. Such alterations correspond to the period of improved integration in language and cognitive processes during adolescence [84].
Taking into account the high plasticity in childhood nervous system, the nature of manifestations may depend on crosstalk between pathological and compensatory mechanisms. For example, in CAE, changes in frontothalamic pathways and associated disorders result in secondary reorganization of adjacent cortical areas allowing for partial compensation in developing functions [86].
On the other hand, current data describe disturbed physiological neuronal epileptogenesis-associated plasticity that was associated with affected events in maturation and formation of brain gray and white matter as well as compensatory potential. For example, the natural development of verbal function is characterized by expanded speech network during maturation, whereas healthy children show more prominent and differentiated development compared to epilepsy children characterized by weak organization and decreased connectivity between verbal functions and other cognitive modules [69]. Such abnormalities can be interpreted as an indicator of putative immaturity and developmental disorders.
Also, one of potential causes for development of cognitive impairment may be due to decreased ability to dynamically restructure a task-driven neural network able to result in altered quality of existing skill implementation [87].
Such age-related characteristics allow for a more dynamic and variable impact from epileptogenic processes on formation of intellectual functions that may also cause varying degree of involvement in cognitive domains in different same disease form patients.
It remains unclear whether the reorganization of neural networks is a sole pathological or compensatory mechanism or whether there is initially an opportunity for a combined interplay between them [65]. Thus, taking into account the existing structural and functional changes patients with CAE and SeLECTS at the time of onset may demonstrate mild or subclinical disorders [31]. In the study by A.N. Datta et al. (2013), children with SeLECTS showed only a tendency toward language impairment. Moreover, the patients had abnormally expanded and strengthened bilateral language networks, whereas physiologically the language network is represented predominantly in the left hemisphere [69].
Mechanisms of cognitive impairment formation in IGE and SeLECTS / Механизмы формирования когнитивных нарушений при ИГЭ и SeLECTS
The described developmental features are interrelated with the age of onset and identified cognitive and anatomical and functional changes observed in various forms of epilepsy. It is argued that SeLECTS may represent a developmental disorder associated with delayed maturation in brain networks [71][88]. Then the causes of diffuse damage with marked speech dysfunction may be genetically determined in preclinical events for developing abnormal neural networks and the early onset of the disease along with focal epileptiform activity in the areas of localized speech function [89].
In the case of IGE, the varying severity of cognitive outcomes among adult patients with diverse disease forms suggests an important role for the age of disease onset in emerging cognitive deficits [18]. However, some studies found no a relationship between the severity of cognitive impairment and IGE form [17].
Indeed, common genetic factors regulating epileptogenesis in IGE are known to be of importance, which can play a decisive role in the predisposition to seizures development as well as remodeling brain substance and disorganizing neural networks observed already at the time of the disease onset. In particular, in JME, one of the mechanisms for developing cognitive deficit relies dysfunction in the thalamofrontal network. It was found that lowered frontal functional activity predominantly affects the severity of the disease manifestations rather than the profile of cognitive impairment [90].
The lack of a predominant affected domain in IGE, as is the case in SeLECTS, points at the involvement of an epileptic focus. Children with SeLECTS show impaired activity in the middle frontal gyrus and superior parietal gyrus, which persists even after decline in centrotemporal spikes [91], which may affect the development of cognitive dysfunction.
Some researchers suggest that the development of absence seizures is not primarily generalized in nature but has a focal onset involving neural networks connecting the ventromedial frontal cortex to the thalamic nuclei, basal ganglia and reticular formation of the brainstem and cerebellum [92]. In this case, the diffuse nature of the lesion may be related to the epileptogenic zones involved in crosstalk and switch of multiple neural networks. For SeLECTS, an opportunity for thalami to directly participate in the generation of epileptic activity has been also discussed [91][93].
The majority data indicate a relationship between specific features of cognitive impairment and brain structural and morphometric alterations in epilepsy [94]. The decline in impulse activity in the central temporal regions and impaired activity of the basal ganglia typical to SeLECTS correlate with the neuroimaging phenotype [88]. Detected bilateral cortical thickening in the precentral regions is considered a sign of delayed brain development and affects not only speech, but also executive function [95].
Structural changes in IGE also have a direct link to the affected cognitive domains. At the same time, according to some data, the neural networks reshaping during IGE solely occurs at suboptimal level. The observed changes with the isolation of functional centers and disrupted links between the limbic system and the basal ganglia are partially compensated by global integrative processes, which, however, are also reduced [67].
The similarity in the localization of functional-anatomical rearrangements and the spectrum of neuropsychological changes suggests the presence of common patterns in the development of cognitive disorders both in IGE and SeLECTS. Qualitative and temporal characteristics of such disorders indicate the important role for preclinical pathological events resulting in an initial deviation in the trajectory of neuropsychic development and formation of diffuse changes.
In the case of SeLECTS, it is impossible to deny the relationship between the focus of epileptiform activity and prevailing speech disorders, but it is problematic to interpret other changes in the cognitive domain as secondary due to the common morpho-functional picture. Persistent multi-impaired cognition in patients with IGE may also indicate an early global impairment in physiological brain maturation and function exacerbated by epileptiform activity and persistent seizures.
CONCLUSION / ЗАКЛЮЧЕНИЕ
The early onset of the pathological process with extensive morpho-functional changes and impaired stages in brain maturation profoundly impact on the further development of cognitive functions in childhood epilepsy patients.
Even if the course is benign and the seizures are stopped, it should be taken into account that epileptogenesis affects neuropsychic development long before overt attacks. The presence of cognitive impairment at the time of onset, together with data on a more severe disease course concomitant with marked intellectual-mnestic disorders allows to consider cognitive disintegration as an important independent link during epileptogenesis in IGE and SeLECTS. It also correlates with the current paradigm shift in the etiological secondary nature of neuropsychological dysfunction in epilepsy.
Taking into account the prevalence and severity of cognitive impairment, it is relevant to determine the tactics for managing such patients and develop a protocol for neuropsychological testing most accessible and informative in everyday clinical practice. Timely diagnostics will allow to identify and adjust potential deviations in the path of developing cognitive functions at the time of onset and organize multidisciplinary crosstalk between medical specialists at early disease stages.
1. NREM – non-rapid eye movement sleep.
References
1. Baumer F.M., Cardon A.L., Porter B.E. Language dysfunction in pediatric epilepsy. J Pediatr. 2018; 194: 13–21. https://doi.org/10.1016/j.jpeds.2017.10.031.
2. Aaberg K.M., Gunnes N., Bakken I.J., et al. Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. 2017; 139 (5): e20163908. https://doi.org/10.1542/peds.2016-3908/38809.
3. Rozensztrauch A., Kołtuniuk A. The quality of life of children with epilepsy and the impact of the disease on the family functioning. Int J Environ Res Public Health. 2022; 19 (4): 2277. https://doi.org/10.3390/ijerph19042277.
4. Sager G., Vatansever Z., Batu U., et al. Neuropsychiatric comorbidities in genetic/idiopathic generalized epilepsies and their effects on psychosocial outcomes. Epilepsy Behav. 2021; 124: 108339. https://doi.org/10.1016/j.yebeh.2021.108339.
5. Kumar G. Evaluation and management of drug resistant epilepsy in children. Curr Probl Pediatr Adolesc Health Care. 2021; 51 (7): 101035. https://doi.org/10.1016/j.cppeds.2021.101035.
6. Sillanpää M., Saarinen M.M., Karrasch M., et al. Neurocognition in childhood epilepsy: impact on mortality and complete seizure remission 50 years later. Epilepsia. 2019; 60 (1): 131–8. https://doi.org/10.1111/epi.14606.
7. Lenck-Santini P.P., Scott R.C. Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harb Perspect Med. 2015; 5 (10): a022772. https://doi.org/10.1101/cshperspect.a022772.
8. Hunter M.B., Yoong M., Sumpter R.E., et al. Neurobehavioral problems in children with early-onset epilepsy: a population-based study. Epilepsy Behav. 2019; 93: 87–93. https://doi.org/10.1016/j.yebeh.2019.01.019.
9. Rogač Ž., Stevanović D., Bečanović S., et al. Cognitive profile, psychopathological symptoms, and quality of life in newly diagnosed pediatric epilepsy: a six-month, naturalistic follow-up study. Epilepsy Res. 2022; 179: 106844. https://doi.org/10.1016/j.eplepsyres.2021.106844.
10. Shalkevich L.V., Zhаuniaronak I.V. Comorbid disorders and their features in children with the manifestation of epilepsy. Int Neurol J. 2019; 6: 5–10. https://doi.org/10.22141/2224-0713.6.108.2019.180529.
11. Blinov D.V. Epilepsy syndromes: the 2022 ILAE definition and classification. Epilepsia i paroksizmalʹnye sostoania / Epilepsy and Paroxysmal Conditions. 2022; 14 (2): 101–82 (in Russ.). https://doi.org/10.17749/2077-8333/epi.par.con.2022.123.
12. Li Y., Sun Y., Niu K., et al. The relationship between neuromagnetic activity and cognitive function in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2020; 112: 107363. https://doi.org/10.1016/j.yebeh.2020.107363.
13. Nilo A., Gelisse P., Crespel A. Genetic/idiopathic generalized epilepsies: not so good as that! Rev Neurol. 2020; 176 (6): 427–38. https://doi.org/10.1016/j.neurol.2020.03.018.
14. Hirsch E., French J., Scheffer I.E., et al. ILAE definition of the idiopathic generalized epilepsy syndromes: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022; 63 (6): 1475–99. https://doi.org/10.1111/epi.17236.
15. Karrasch M., Tiitta P., Hermann B., et al. Cognitive outcome in childhood-onset epilepsy: a five-decade prospective cohort study. J Int Neuropsychol Soc. 2017; 23 (4): 332–40. https://doi.org/10.1017/S1355617716001077.
16. Menlove L., Reilly C. Memory in children with epilepsy: a systematic review. Seizure. 2015; 25: 126–35. https://doi.org/10.1016/j.seizure.2014.10.002.
17. Loughman A., Bowden S.C., D’Souza W. Cognitive functioning in idiopathic generalised epilepsies: a systematic review and metaanalysis. Neurosci Biobehav Rev. 2014; 43: 20–34. https://doi.org/10.1016/j.neubiorev.2014.02.012.
18. Abarrategui B., Parejo-Carbonell B., García García M.E., et al. The cognitive phenotype of idiopathic generalized epilepsy. Epilepsy Behav. 2018; 89: 99–104. https://doi.org/10.1016/j.yebeh.2018.10.007.
19. Hermann B.P., Zhao Q., Jackson D.C., et al. Cognitive phenotypes in childhood idiopathic epilepsies. Epilepsy Behav. 2016; 61: 269–74. https://doi.org/10.1016/j.yebeh.2016.05.013.
20. Germanò E., Gagliano A., Arena C., et al. Reading–writing disorder in children with idiopathic epilepsy. Epilepsy Behav. 2020; 111: 107118. https://doi.org/10.1016/j.yebeh.2020.107118.
21. Cheng D., Yan X., Xu K., et al. The effect of interictal epileptiform discharges on cognitive and academic performance in children with idiopathic epilepsy. BMC Neurol. 2020; 20 (1): 233. https://doi.org/10.1186/s12883-020-01807-z/tables/3.
22. Lin J.J., Dabbs K., Riley J.D., et al. Neurodevelopment in new-onset juvenile myoclonic epilepsy over the first 2 years. Ann Neurol. 2014; 76 (5): 660–8. https://doi.org/10.1002/ana.24240.
23. Cheng D., Yan X., Gao Z., et al. Common and distinctive patterns of cognitive dysfunction in children with benign epilepsy syndromes. Pediatr Neurol. 2017; 72: 36–41.e1. https://doi.org/10.1016/j.pediatrneurol.2016.12.005.
24. Jackson D.C., Dabbs K., Walker N.M., et al. The neuropsychological and academic substrate of new-onset epilepsies. J Pediatr. 2013; 162 (5): 1047. https://doi.org/10.1016/j.jpeds.2012.10.046.
25. Cheng D., Yan X., Gao Z., et al. Neurocognitive profiles in childhood absence epilepsy. J Child Neurol. 2017; 32 (1): 46–52. https://doi.org/10.1177/0883073816668465.
26. Masur D., Shinnar S., Cnaan A., et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology. 2013; 81 (18): 1572–80. https://doi.org/10.1212/wnl.0b013e3182a9f3ca.
27. Caciagli L., Ratcliffe C., Xiao F., et al. The cognitive phenotype of juvenile absence epilepsy and its heritability: an investigation of patients and unaffected siblings. medRxiv. 2022.04.12.22273461. https://doi.org/10.1101/2022.04.12.22273461.
28. Kim E.H., Ko T.S. Cognitive impairment in childhood onset epilepsy: up-to-date information about its causes. Korean J Pediatr. 2016; 59 (4): 155. https://doi.org/10.3345/kjp.2016.59.4.155.
29. Almane D.N., Jones J.E., McMillan T., et al. The timing, nature, and range of neurobehavioral comorbidities in juvenile myoclonic epilepsy. Pediatr Neurol. 2019; 101: 47–52. https://doi.org/10.1016/j.pediatrneurol.2019.03.011.
30. Kwon S., Seo H.E., Hwang S.K. Cognitive and other neuropsychological profiles in children with newly diagnosed benign rolandic epilepsy. Korean J Pediatr. 2012; 55 (10): 383. https://doi.org/10.3345/kjp.2012.55.10.383.
31. Wickens S., Bowden S.C., D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: a systematic review and meta-analysis. Epilepsia. 2017; 58 (10): 1673–85. https://doi.org/10.1111/epi.13865.
32. Smith A.B., Bajomo O., Pal D.K. A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol. 2015; 57 (11): 1019–26. https://doi.org/10.1111/dmcn.12856.
33. Yang Y., Wang F., Andrade-Machado R., et al. Disrupted functional connectivity patterns of the left inferior frontal gyrus subregions in benign childhood epilepsy with centrotemporal spikes. Transl Pediatr. 2022; 11 (9): 1552–61. https://doi.org/10.21037/tp-22-270/coif.
34. Verrotti A., Filippini M., Matricardi S., et al. Memory impairment and benign epilepsy with centrotemporal spike (BECTS): a growing suspicion. Brain Cogn. 2014; 84 (1): 123–31. https://doi.org/10.1016/j.bandc.2013.11.014.
35. Lopes A.F., Monteiro J.P., Fonseca M.J., et al. Memory functioning in children with epilepsy: frontal lobe epilepsy, childhood absence epilepsy, and benign epilepsy with centrotemporal spikes. Behav Neurol. 2014; 2014: 218637. https://doi.org/10.1155/2014/218637.
36. Chowdhury F.A., Elwes R.D.C., Koutroumanidis M., et al. Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: an epilepsy endophenotype. Epilepsia. 2014; 55 (6): 835–40. https://doi.org/10.1111/epi.12604/supinfo.
37. Stier C., Loose M., Kotikalapudi R., et al. Combined electrophysiological and morphological phenotypes in patients with genetic generalized epilepsy and their healthy siblings. Epilepsia. 2022; 63 (7): 1643–57. https://doi.org/10.1111/epi.17258.
38. Verrotti A., Matricardi S., Di Giacomo D.L., et al. Neuropsychological impairment in children with Rolandic epilepsy and in their siblings. Epilepsy Behav. 2013; 28 (1): 108–12. https://doi.org/10.1016/j.yebeh.2013.04.005.
39. Gesche J., Beier C.P. Drug resistance in idiopathic generalized epilepsies: evidence and concepts. Epilepsia. 2022; 63 (12): 3007–19. https://doi.org/10.1111/epi.17410.
40. Riaz M., Abbasi M.H., Sheikh N., et al. GABRA1 and GABRA6 gene mutations in idiopathic generalized epilepsy patients. Seizure. 2021; 93: 88–94. https://doi.org/10.1016/j.seizure.2021.10.013.
41. Perucca P., Bahlo M., Berkovic S.F. The genetics of epilepsy. Annu Rev Genomics Hum Genet. 2020; 21: 205–30. https://doi.org/10.1146/annurev-genom-120219-074937.
42. Thakran S., Guin D., Singh P., et al. Genetic landscape of common epilepsies: advancing towards precision in treatment. Int J Mol Sci. 2020; 21 (20): 7784. https://doi.org/10.3390/ijms21207784.
43. Rinaldi V.E., Di Cara G., Mencaroni E., Verrotti A. Therapeutic options for childhood absence epilepsy. Pediatr Rep. 2021; 13 (4): 658–67. https://doi.org/10.3390/pediatric13040078.
44. Yalin Ö. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure. 2012; 21 (2): 79–86. https://doi.org/10.1016/j.seizure.2011.12.002.
45. Gilsoul M., Grisar T., Delgado-Escueta A.V., et al. Subtle brain developmental abnormalities in the pathogenesis of juvenile myoclonic epilepsy. Front Cell Neurosci. 2019; 13: 465519. https://doi.org/10.3389/fncel.2019.00433.
46. Timechko E.E., Shilkina O.S., Oreshkova N.V., et al. Whole-exome sequencing of patients with juvenile myoclonic epilepsy. Epilepsia i paroksizmalʹnye sostoania / Epilepsy and Paroxysmal Conditions. 2022; 14 (3): 254–66 (in Russ.). https://doi.org/10.17749/2077-8333/epi.par.con.2022.119.
47. Shilkina O.S., Zobova S.N., Domoratskaya E.A., Dmitrenko D.V. Clinical and genetic characteristics of juvenile myoclonic epilepsy. Personalized Psychiatry and Neurology. 2021; 1 (2): 95–105. https://doi.org/10.52667/2712-9179-2021-1-2-95-105.
48. Shilkina O.S., Shnayder N.A., Zobova S.N., et al. Association of the carriage of BRD2 rs206787 and rs516535 and GJD2 rs3743123 polymorphisms with juvenile myoclonic epilepsy in Caucasian patients of Siberia. Neurology, Neuropsychiatry, Psychosomatics. 2019; 11 (4): 61–7 (in Russ.). https://doi.org/10.14412/2074-2711-2019-4-61-67.
49. Xiong W., Zhou D. Progress in unraveling the genetic etiology of rolandic epilepsy. Seizure. 2017; 47: 99–104. https://doi.org/10.1016/j.seizure.2017.02.012.
50. Li X., Jiang L. Research progress in genetic studies of Rolandic epilepsy. Chinese Journal of Applied Clinical Pediatrics. 2020; 24: 314–7. https://doi.org/10.3760/cma.j.cn101070-20190306-00167.
51. Shi X.Y., Wang G., Li T., et al. Identification of susceptibility variants to benign childhood epilepsy with centro-temporal spikes (BECTS) in Chinese Han population. EBioMedicine. 2020; 57: 102840. https://doi.org/10.1016/j.ebiom.2020.102840.
52. Ma M.G., Liu X.R., Wu Y., et al. RYR2 mutations are associated with benign epilepsy of childhood with centrotemporal spikes with or without arrhythmia. Front Neurosci. 2021; 15: 629610. https://doi.org/10.3389/FNINS.2021.629610.
53. Byars A.W., deGrauw T.J., Johnson C.S., et al. Language and social functioning in children and adolescents with epilepsy. Epilepsy Behav. 2014; 31: 167–71. https://doi.org/10.1016/j.yebeh.2013.11.007.
54. Egunsola O., Choonara I., Sammons H.M., Whitehouse W.P. Safety of antiepileptic drugs in children and young people: a prospective cohort study. Seizure. 2018; 56: 20–5. https://doi.org/10.1016/j.seizure.2018.01.018.
55. Mohamed I.N., Osman A.H., Mohamed S., et al. Intelligence quotient (IQ) among children with epilepsy: national epidemiological study – Sudan. Epilepsy Behav. 2020; 103: 106813. https://doi.org/10.1016/j.yebeh.2019.106813.
56. Azad C., Guglani V., Siddiqui A., et al. Psychopathological aspects in children with epilepsy and its contributing factors: a cross-sectional study from India. J Neurosci Rural Pract. 2022; 13 (2): 301–6. https://doi.org/10.1055/s-0042-1743459.
57. Fujiwara H., Tenney J., Kadis D.S., et al. Cortical and subcortical volume differences between benign epilepsy with centrotemporal spikes and childhood absence epilepsy. Epilepsy Res. 2020; 166: 106407. https://doi.org/10.1016/j.eplepsyres.2020.106407.
58. Si X., Zhang X., Zhou Y., et al. White matter structural connectivity as a biomarker for detecting juvenile myoclonic epilepsy by transferred deep convolutional neural networks with varying transfer rates. J Neural Eng. 2021; 18 (5): 056053. https://doi.org/10.1088/1741-2552/ac25d8.
59. Kim E.H., Yum M.S., Shim W.H., et al. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure. 2015; 27: 40–6. https://doi.org/10.1016/j.seizure.2015.02.027.
60. Garcia-Ramos C., Jackson D.C., Lin J.J., et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015; 56 (10): 1615–22. https://doi.org/10.1111/epi.13125.
61. Suzuki T., Inoue I., Yamakawa K. Epilepsy protein Efhc1/myoclonin1 is expressed in cells with motile cilia but not in neurons or mitotic apparatuses in brain. Sci Rep. 2020; 10 (1): 22076. https://doi.org/10.1038/s41598-020-79202-4.
62. Royer J., Bernhardt B.C., Larivière S., et al. Epilepsy and brain network hubs. Epilepsia. 2022; 63 (3): 537–50. https://doi.org/10.1111/epi.17171.
63. Pegg E.J., Taylor J.R., Keller S.S., Mohanraj R. Interictal structural and functional connectivity in idiopathic generalized epilepsy: a systematic review of graph theoretical studies. Epilepsy Behav. 2020; 106: 107013. https://doi.org/10.1016/j.yebeh.2020.107013.
64. Wu Y., Ji G.J., Zang Y.F., et al. Local activity and causal connectivity in children with benign epilepsy with centrotemporal spikes. PLoS One. 2015; 10 (7): e0134361. https://doi.org/10.1371/journal.pone.0134361.
65. Kellermann T.S., Bonilha L., Lin J.J., Hermann B.P. Mapping the landscape of cognitive development in children with epilepsy. Cortex. 2015; 66: 1–8. https://doi.org/10.1016/j.cortex.2015.02.001.
66. Jiang S., Pei H., Huang Y., et al. Dynamic temporospatial patterns of functional connectivity and alterations in idiopathic generalized epilepsy. Int J Neural Syst. 2020; 30 (12): 2050065. https://doi.org/10.1142/S0129065720500653.
67. Zhang T., Zhang Y., Ren J., et al. Aberrant basal ganglia-thalamocortical network topology in juvenile absence epilepsy: a resting-state EEG-fMRI study. Seizure. 2021; 84: 78–83. https://doi.org/10.1016/j.seizure.2020.11.015.
68. Barone V., van Putten M.J., Visser G.H. Absence epilepsy: characteristics, pathophysiology, attention impairments, and the related risk of accidents. A narrative review. Epilepsy Behav. 2020; 112: 107342. https://doi.org/10.1016/j.yebeh.2020.107342.
69. Datta A.N., Oser N., Bauder F., et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013; 54 (3): 487–94. https://doi.org/10.1111/epi.12067.
70. Li R., Liao W., Yu Y., et al. Differential patterns of dynamic functional connectivity variability of striato–cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. 2018; 39 (3): 1207–17. https://doi.org/10.1002/hbm.23910.
71. Besseling R.M., Jansen J.F., Overvliet G.M., et al. Delayed convergence between brain network structure and function in rolandic epilepsy. Front Hum Neurosci. 2014; 8: 704. https://doi.org/10.3389/fnhum.2014.00704.
72. Besseling R.M., Jansen J.F., Overvliet G.M., et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. Neuroimage Clin. 2013; 2 (1): 239–46. https://doi.org/10.1016/j.nicl.2013.01.004.
73. Ito Y., Maki Y., Okai Y., et al. Involvement of brain structures in childhood epilepsy with centrotemporal spikes. Pediatr Int. 2022; 64 (1): e15001. https://doi.org/10.1111/ped.15001.
74. Jeong M.H., Yum M.S., Ko T.S., et al. Neuropsychological status of children with newly diagnosed idiopathic childhood epilepsy. Brain Dev. 2011; 33 (8): 666–71. https://doi.org/10.1016/j.braindev.2010.11.003.
75. Vaudano A.E., Avanzini P., Cantalupo G., et al. Mapping the effect of interictal epileptic activity density during wakefulness on brain functioning in focal childhood epilepsies with centrotemporal spikes. Front Neurol. 2019; 10: 486513. https://doi.org/10.3389/fneur.2019.01316.
76. Vannest J., Tenney J.R., Gelineau-Morel R., et al. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2015; 45: 85–91. https://doi.org/10.1016/j.yebeh.2015.01.041.
77. Zhang J., Yang H., Wu D., et al. Electroencephalographic abnormalities are correlated with cognitive deficits in children with benign childhood epilepsy with centrotemporal spikes: a clinical study of 61 cases. Epilepsy Behav. 2020; 106: 107012. https://doi.org/10.1016/j.yebeh.2020.107012.
78. Li R., Ji G.J., Yu Y., et al. Epileptic discharge related functional connectivity within and between networks in benign epilepsy with centrotemporal spikes. Int J Neural Syst. 2017; 27 (7): 1750018. https://doi.org/10.1142/S0129065717500186.
79. Balcik Z.E., Senadim S., Tekin B., et al. Do interictal EEG findings reflect cognitive function in juvenile myoclonic epilepsy? Epilepsy Behav. 2020; 111: 107281. https://doi.org/10.1016/j.yebeh.2020.107281.
80. Sezikli S., Pulat T.A., Tekin B., et al. Frontal lobe cognitive functions and electroencephalographic features in juvenile myoclonic epilepsy. Epilepsy Behav. 2018; 86: 102–7. https://doi.org/10.1016/j.yebeh.2018.06.009.
81. Dharan A.L., Bowden S.C., Peterson A., et al. A cross-sectional investigation of cognition and epileptiform discharges in juvenile absence epilepsy. Epilepsia. 2023; 64 (3): 742–53. https://doi.org/10.1111/epi.17505.
82. Dlugos D., Shinnar S., Cnaan A., et al. Pretreatment EEG in childhood absence epilepsy. Neurology. 2013; 81 (2): 150–6. https://doi.org/10.1212/wnl.0b013e31829a3373.
83. Hermann B.P., Struck A.F., Busch R.M., et al. Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy. Nat Rev Neurol. 2021; 17 (12): 731–46. https://doi.org/10.1038/s41582-021-00555-z.
84. Addis L., Lin J.J., Pal D.K., et al. Imaging and genetics of language and cognition in pediatric epilepsy. Epilepsy Behav. 2013; 26 (3): 303–12. https://doi.org/10.1016/j.yebeh.2012.09.014.
85. Shaw P., Kabani N.J., Lerch J.P., et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008; 28 (14): 3586–94. https://doi.org/10.1523/jneurosci.5309-07.2008.
86. Verrotti A., Matricardi S., Rinaldi V.E., et al. Neuropsychological impairment in childhood absence epilepsy: review of the literature. J Neurol Sci. 2015; 359 (1-2): 59–66. https://doi.org/10.1016/j.jns.2015.10.035.
87. Tailby C., Kowalczyk M.A., Jackson G.D. Cognitive impairment in epilepsy: the role of reduced network flexibility. Ann Clin Transl Neurol. 2018; 5 (1): 29–40. https://doi.org/10.1002/acn3.503.
88. Zhang Q., He Y., Qu T., et al. Delayed brain development of Rolandic epilepsy profiled by deep learning-based neuroanatomic imaging. Eur Radiol. 2021; 31 (12): 9628–37. https://doi.org/10.1007/S00330-021-08048-9.
89. Zhang Q., Li J., He Y., et al. Atypical functional connectivity hierarchy in Rolandic epilepsy. Commun Biol. 2023; 6: 704. https://doi.org/10.1038/s42003-023-05075-8.
90. Kim J.H., Suh S.I., Park S.Y., et al. Microstructural white matter abnormality and frontal cognitive dysfunctions in juvenile myoclonic epilepsy. Epilepsia. 2012; 53 (8): 1371–8. https://doi.org/10.1111/j.1528-1167.2012.03544.x.
91. Zhu Y., Yu Y., Shinkareva S.V., et al. Intrinsic brain activity as a diagnostic biomarker in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. 2015; 36 (10): 3878–89. https://doi.org/10.1002/hbm.22884.
92. Depaulis A., Charpier S. Pathophysiology of absence epilepsy: insights from genetic models. Neurosci Lett. 2018; 667: 53–65. https://doi.org/10.1016/j.neulet.2017.02.035.
93. Li Q., Westover M.B., Zhang R., Chu C.J. Computational evidence for a competitive thalamocortical model of spikes and spindle activity in Rolandic epilepsy. Front Comput Neurosci. 2021; 15: 680549. https://doi.org/10.3389/fncom.2021.680549.
94. Garcia-Ramos C., Lin J.J., Kellermann T.S., et al. Graph theory and cognition: a complementary avenue for examining neuropsychological status in epilepsy. Epilepsy Behav. 2016; 64: 329–35. https://doi.org/10.1016/j.yebeh.2016.02.032.
95. Xu Y., Xu Q., Zhang Q., et al. Influence of epileptogenic region on brain structural changes in Rolandic epilepsy. Brain Imaging Behav. 2022; 16 (1): 424–34. https://doi.org/10.1007/s11682-021-00517-5.
About the Authors
A. I. ParamonovaRussian Federation
Anastasia I. Paramonova – Postgraduate, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Postgraduate Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: HMP-3496-2023
K. D. Lysova
Russian Federation
Kristina D. Lysova – Assistant Professor, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Postgraduate Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
E. E. Timechko
Russian Federation
Elena E. Timechko – Junior Researcher, Laboratory of Medical Genetics, Center for Collective Use “Molecular and Cellular Technologies”
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: CAF-2677-2022
G. V. Senchenko
Russian Federation
Galina V. Senchenko – Senior Lecturer, Chair of Clinical Psychology and Pedagogy with a Course of Postgraduate Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: AAO-2210-2020
M. R. Sapronova
Russian Federation
Margarita R. Sapronova – MD, PhD, Associate Professor, Chair of Medical Genetics and Clinical Neurophysiology, Institute of Postgraduate Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: AAJ-2507-2020
D. V. Dmitrenko
Russian Federation
Diana V. Dmitrenko – Dr. Med. Sc., Chief of Chair of Medical Genetics and Clinical Neurophysiology, Institute of Postgraduate Education
1 Partizan Zheleznyak Str., Krasnoyarsk 660022, Russia
WoS ResearcherID: H-7787-2016
Review
For citations:
Paramonova A.I., Lysova K.D., Timechko E.E., Senchenko G.V., Sapronova M.R., Dmitrenko D.V. Cognitive impairment in childhood-onset epilepsy. Epilepsy and paroxysmal conditions. 2024;16(1):54-68. https://doi.org/10.17749/2077-8333/epi.par.con.2024.176
JATS XML

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.










































